Contribution of Metabolic Tumor Volume and Total Lesion Glycolysis to Predict Prognosis in Early-Stage Lung Cancer at Preoperative Staging
By Elanur Karaman1, Sibel Goksel2, Kerim Tuluce3Affiliations
doi: 10.29271/jcpsp.2022.06.740ABSTRACT
Objective: To evaluate the effect of metabolic tumor volume (MTV) and total lesion glycolysis (TLG) values of the primary tumor measured by preoperative positron emission tomography/computed tomography (FDG-PET/CT) on survival in patients with operated non-small cell lung cancer (NSCLC).
Study Design: Cohort study.
Place and Duration of Study: Recep Tayyip Erdogan University, Faculty of Medicine, Department of Medical Oncology from January 2017 to June 2020, Turkey.
Methodology: Patients with operated NSCLC were reviewed retrospectively. Metabolic parameters of FDG-PET/CT such as pathological tumor features, type of operation, MTV/TLG values, and whether they received adjuvant therapy were evaluated. Disease-free survival (DFS) and overall survival (OS) times were calculated.
Results: Most of the 77 patients (96.1%) were male. The mean age is 64±8 years. Lobectomy was performed in 66 (85.7%) patients, and pneumonectomy was performed in 11 (14.3%) patients. The mean tumor diameter was 3.7±2.015cm. Squamous cell carcinoma was detected in 37 patients (48.1%) and adenocarcinoma in 35 patients (45.5%). Thirty-eight patients (49.4%) received adjuvant chemotherapy. SUVmax, MTV, and TLG values of the primary tumor were high in patients under 65 years of age and with a tumor diameter of ≥3cm. DFS was nine months (4.5-18), and OS was 19 months (11-29). The 2-year survival rate was 75.6%. It was observed that patients with adenocarcinoma relapsed more frequently, which negatively affected survival (p=0.023, and p=0.024 respectively). High MTV (p=0.01) and TLG (p=0.015) values were associated with poor prognosis.
Conclusion: NSCLC is a heterogeneous disease, and survival is affected by many factors. Our study showed that the subtype of adenocarcinoma and high MTV and TLG values of the primary tumor are poor prognostic factors in operated early-stage lung cancers.
Key Words: Early-stage, Non-small cell lung cancer, MTV, TLG, NSCLC, Survival, FDG-PET/CT.
INTRODUCTION
Just about a third of lung cancer, which is the most important cause of cancer-related mortality, can be detected at an early-stage. The primary treatment for early stage non-small cell lung cancer (NSCLC) is surgery.1 Despite this, the disease recurs in 55-70% of patients. Factors such as TNM staging, histological type, smoking, comorbidities, ECOG performance status, and receiving treatment affect the prognosis.
Despite being at the same stage, survival times and response rates to treatment may differ in some patients.2,3 Many studies suggest that TNM used in staging does not always give accurate results, and thus survival is affected by many tumors and patient-specific heterogeneous factors.4
Fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG-PET/CT) is an imaging method that is becoming increasingly common all over the world in the staging of many cancers, based on the FDG uptake of metabolically active cells. It allows scanning the whole body in a single session and is most commonly used in oncology practice. It has been frequently used to evaluate lung cancer diagnosis, staging, restaging, and treatment response and is included in the guidelines.5 While maximum standard uptake value (SUVmax) reflects the metabolic status of the tumor, metabolic tumor volume (MTV) and total lesion glycolysis (TLG) are parameters that reflect both metabolic and volumetric status.6 It has been reported that high MTV and TLG levels in lung cancer may be prognostic factors in stipulating disease-free survival (DFS) and overall survival (OS).7 Despite advances in diagnosis and treatment modalities, the 5-year survival rate is 50%, even in patients who underwent surgery.6 It is crucial to determine the prognostic factors that can predict patients with a high risk of relapse in early-stage NSCLC patients, where survival has not yet been brought to the desired levels.5
In this study, ıt was aimed to appreciate the effect of metabolic parameters of FDG-PET/CT such as SUVmax, MTV, and TLG of the primary tumor on survival in patients with early-stage NSCLC who had undergone surgery.
METHODOLOGY
Before starting the study, ethical approval was obtained from the Ethics Committee of Recep Tayyip Erdoğan University Faculty of Medicine (Date: 24.06.2020, no: 2020/122). The study was managed according to the Helsinki principles. NSCLC patients with operated who applied to the Medical Oncology outpatient clinic of the University Hospital between 2017 to 2020 were evaluated retrospectively. Patients aged >18 years with preoperative FDG-PET/CT images and who had undergone surgery for first-line treatment of the primary tumor were included in the study. Patients with synchronous or metachronous tumors, stage IV disease, and those who did not undergo preoperative FDG-PET/CT imaging, receiving neoadjuvant therapy prior to initial FDG-PET/CT scan were excluded from the study. Clinical features of the patients, tumor location, histopathological subtype of NSCLC, date of diagnosis, type of operation, two-dimensional tumor diameter on FDG-PET/CT scan, visceral pleural invasion, presence of perineural invasion (PNI) and lymphovascular invasion (LVI), pathological tumor grade, preoperative SUVmax, SUVmean, MTV and TLG values of the primary tumor on the FDG-PET/CT scanning, whether they received adjuvant treatment after the surgery, and whether they developed recurrence during the follow-up of the patients were recorded. DFS was calculated as the time from diagnosis to recurrence, and OS was calculated as the time from diagnosis to death or last follow-up.
At least 6 hours of fasting was requested from the patients before the FDG injection. The fasting blood glucose levels of all the patients were <200 mg/dL before scanning. Approximately 220-370 MBq dose of 18F-FDG was given intravenously to the patients. An oral contrast agent was given to all patients. The patients were subjected to the FDG-PET/CT (Siemens Biograph mCT, 20 slices) with 3D mode and TOF features, following a resting period of 50–70 minutes in the waiting room. Images were obtained from the head to the upper thigh region. A low dose CT scan was collected at an average of 120 kV and 50 mAs for attenuation correction of FDG-PET images. The FDG-PET acquisition was obtained at the rate of 2 minutes per bed position. All FDG-PET/CT images of the patients were re-evaluated by nuclear medicine physician a board-certified. SUVmax, SUVmean, MTV, TLG values were calculated automatically in the software program in the primary tumor plotted volume of interest (VOI) area. No measurements were made to the lymph nodes. The threshold value used in MTV calculation was taken as 40% of SUVmax and above, and metabolically active tumor volume was calculated.
IBM © SPSS program version 20 was used for the analyses. In the study, descriptive data were shown as n and % values in categorical data and median [(IQR: Interquartile range (25-75 percentile values)] values in continuous data. Chi-square and Fisher tests were used to compare categorical data. Whether the data were normally distributed or not was evaluated with the Kolmogorov-Smirnov test. Mann-Whitney U-test and Kruskal Wallis tests were used for data that did not show normal distribution. Spearman and Pearson correlation analyses were used for correlation analysis. The Kaplan Meier test was used in the survival analysis of the patients, while the factors affecting the survival time were evaluated with the Cox regression analysis. P<0.05 was accepted for statistical significance in all analyzes.
RESULTS
A total of 77 patients mean age 64.3±8 with surgery history of lung cancer, most of whom were male 74 (96.1%) and 3 (3.9%) were female were included in the study. When the difference in the metabolic parameters of the primary tumor between the patients with and without recurrence was examined, it was found that MTV, and TLG values were higher in patients with recurrence. However, SUVmax-SUV mean values were similar in both groups, and no significant difference was found. Demographic and clinical data of all patients according to recurrence status are summarised in Table I.
Right upper and middle lobectomy to 28 patients (36.4%), left upper lobectomy to 18 (23.4%) patients, right lower lobectomy to 12 (15.6%) patients, the left lower lobectomy to 8 (10.4%) patients was performed. Pneumonectomy was performed on 11 (14.3%) patients. While 30 patients (39%) were Stage 1, 25 patients (32.5%) were Stage 2 and 22 patients (28.6%) were Stage 3. No statistically significant difference was determined when metabolic parameters of FDG-PET/CT (SUVmax, SUVmean, MTV, TLG) were analyzed according to stages. Recurrence was detected in 15 patients (19.5%), and metastasis was found in 18 patients (23.4%). It was observed that 39 patients (50.6%) received adjuvant chemotherapy, 7 (9.1%) patients received adjuvant chemotherapy and radiotherapy, and 31 patients (40.3%) did not receive adjuvant therapy.
When the relationship between metabolic tumor parameters and age, tumor diameter, presence, and the number of the metastatic lymph node are examined; SUVmax and TLG were negatively correlated with age (r=-0.261, p=0.022; r=-0.224, p=0.064, respectively), SUVmax, MTV, and TLG were positively correlated with tumor diameter (r=0.340, p=0.002; r=0.676, p<0.001, r=0.699, p=<0.001, respectively). It was observed that only TLG was positively correlated with the total number of metastatic lymph nodes (r=0.243, p=0.048). There was no significant correlation between the presence of metastatic lymph nodes and metabolic tumor parameters (Table II).
Table I: Demographic and clinical characteristics of patients.
|
|
Total (%) n=77(100) |
Recurrence or metastasis (%) n= 24 (31.2) |
No Recurrence or metastasis (%) n= 53 (68.8) |
p |
|
Age (mean or median) |
64.28±7.9 |
60.5 (55.5-68.5) |
66 (60-70) |
0.176a |
|
Gender Male Female |
74 (96.1) 3 (3.9) |
22 (91.6) 2 (8.3) |
52 (98.1) 1 (1.8) |
0.228b |
|
Histopathological Subgroup Adenocarcinoma Squamous Cell Carcinoma Others |
35 (38.9) 37 (48.0) 5 (6.5) |
16 (66.6) 7 (29.1) 1 (4.1) |
19 (35.8) 30 (56.6) 4 (7.5) |
0.023b |
|
Tumor size (mm, median) |
37 (10-90) |
35.5 (10-85) |
38 (15-90) |
0.741a |
|
Lemphovascular invasion, n:76 (+) (-) |
23 (30.2) 53 (69.7) |
5 (20.8) 19 (79.1) |
18 (34.6) 34 (65.3) |
0.224c |
|
Perineural invasion, n:73 (+) (-) |
17 (23.2) 56 (76.7) |
3 (13.0) 20 (86.9) |
14 (28.0) 36 (72.0) |
0.160c |
|
Surgical margin, n:76 (+) (-) |
6 (7.9) 70 (92.1) |
3 (13.0) 20 (86.9) |
3 (5.6) 50 (94.3) |
0.359c |
|
Plevral invasion, n:75 (+) (-) |
31 (41.3) 44 (58.7) |
8 (34.7) 15 (65.2) |
23 (44.2) 29 (55.7) |
0.444c |
|
Metabolic parameters of primary tumor SUVmax (median) SUVmean (median) MTV (cm3, median) TLG (g, median) |
12 (8.1-17.8) 7.7 (5.0-11.1) 12.3 (4.9-32.1) 107.3 (25.5-312.2) |
12.1 (8.7-17.7) 7.7 (4.5-11.0) 14.8 (6.1-51.6) 102.9 (29.8-396.3) |
12.0 (8.1-19.3) 7.7 (5-11.5) 11.5 (4.1-31.3) 107.9 (23.3-249.8) |
0.939a 0.722a 0.390a 0.703a |
|
Type of Surgery Lobectomy Pneumonectomy |
66 (85.7) 11 (14.2) |
22 (91.6) 2 (8.3) |
44 (83.0) 9 (16.9) |
0.265b |
|
Adjuvant Treatment Yes (CT/CT+RT)* No |
46 (59.7) 31 (40.3) |
16 (66.6) 8 (33.3) |
30 (56.6) 23 (43.3) |
0.404c |
|
Overall Survival (OS) (month) |
19 (11-29) |
37.2 (26.2-48.1) |
87.6 (76.5-98.7) |
0.003d |
|
IQR: Interquartile range (25-75 percentile values), a Mann Whitney U-test, bFisher test, cKi kare test, dKaplan-Meier test *Recurrence or metastasis status could not be determined in 3 patients who underwent lobectomy **CT: Chemotherapy, RT: Radiotherapy. |
||||
Table II: Correlation between metabolic tumor parameters with age, tumor diameter, presence of lymph node metastases, and number of metastatic lymph nodes.
|
|
Age |
Tumor size |
Presence of metastatic lymph nodes |
Total metastatic lymph nodes |
||||
|
Rho |
p |
Rho |
p |
Rho |
p |
Rho |
p |
|
|
SUVmax |
-0.261 |
0.022 |
0.340 |
0.002 |
0.222 |
0.054 |
0.039 |
0.740 |
|
MTV (cm3) |
-0.175 |
0.151 |
0.676 |
<0.001 |
0.018 |
0.882 |
0.180 |
0.145 |
|
TLG (g) |
-0.224 |
0.064 |
0.699 |
<0.001 |
0.080 |
0.515 |
0.243 |
0.048 |
|
* Sperman korelasyon analizi; **SUVmax: Maximum standart uptake value, MTV: Metabolic tumor volmue, TLG: Total lesion glycolysis. |
||||||||
When the survival times were examined, the OS was 19 months (11-29), and the DFS was 9 months (4.5-18). In the follow-ups, 23 patients (29.9%) dead. No significant correlation was observed between the metabolic parameters of the primary tumor and the development of recurrence or metastasis. It was observed that patients with the adenocarcinoma histological subtype relapsed or metastasized more frequently than other histological types (p=0.023). OS was lower in patients with adenocarcinoma histology who developed recurrence or metastasis than in other histologic types (p=0.024; Figure 1).
When recurrence developed in patients who had more than six lymph nodes removed during the operation, it was determined that they lived longer than those who had less than six lymph nodes removed (82.4 vs. 28.3 months p=0.016).
Table III: Multivariate analyses of clinicopathological and metabolic parameters of FDG-PET/CT predictive of prognosis.
|
|
HR |
Model A (%95 GA) |
p |
HR |
Model B (%95 GA) |
p |
||
|
Lower |
Upper |
Lower |
Upper |
|||||
|
Age |
1.08 |
0.97 |
1.20 |
0.166 |
1.09 |
0.99 |
1.21 |
0.093 |
|
Gender (male) |
1.00 |
0.14 |
7.31 |
0.998 |
|
|
|
|
|
SUVmax |
1.30 |
0.81 |
2.09 |
0.285 |
|
|
|
|
|
SUVmean |
0.55 |
0.24 |
1.26 |
0.156 |
0.82 |
0.67 |
1.02 |
0.069 |
|
MTV (cm3) |
0.93 |
0.88 |
0.99 |
0.014 |
0.94 |
0.90 |
0.99 |
0.010 |
|
TLG (g) |
1.01 |
1.00 |
1.02 |
0.022 |
1.01 |
1.00 |
1.01 |
0.015 |
|
Grade-1 (Reference) |
||||||||
|
Grade-2 |
0.02 |
0.00 |
0.17 |
0.000 |
0.05 |
0.01 |
0.29 |
0.001 |
|
Grade-3 |
0.18 |
0.02 |
1.74 |
0.138 |
0.30 |
0.06 |
1.43 |
0.131 |
|
Recurrence or metastases |
12.40 |
2.98 |
51.55 |
0.001 |
7.00 |
2.15 |
22.84 |
0.001 |
|
Visceral pleural invasion (+) |
2.20 |
0.61 |
7.96 |
0.228 |
|
|
|
|
|
Surgical margin (+) |
0.32 |
0.05 |
2.02 |
0.225 |
|
|
|
|
|
*Model A: model consisting of all the variables to be corrected, Model B: Optimal model; **SUVmax: Maximum standard uptake value, SUVmean: Mean standard uptake value, MTV: Metabolic tumor volume, TLG: Total lesion glycolysis, HR: Hazard Ratio. |
||||||||
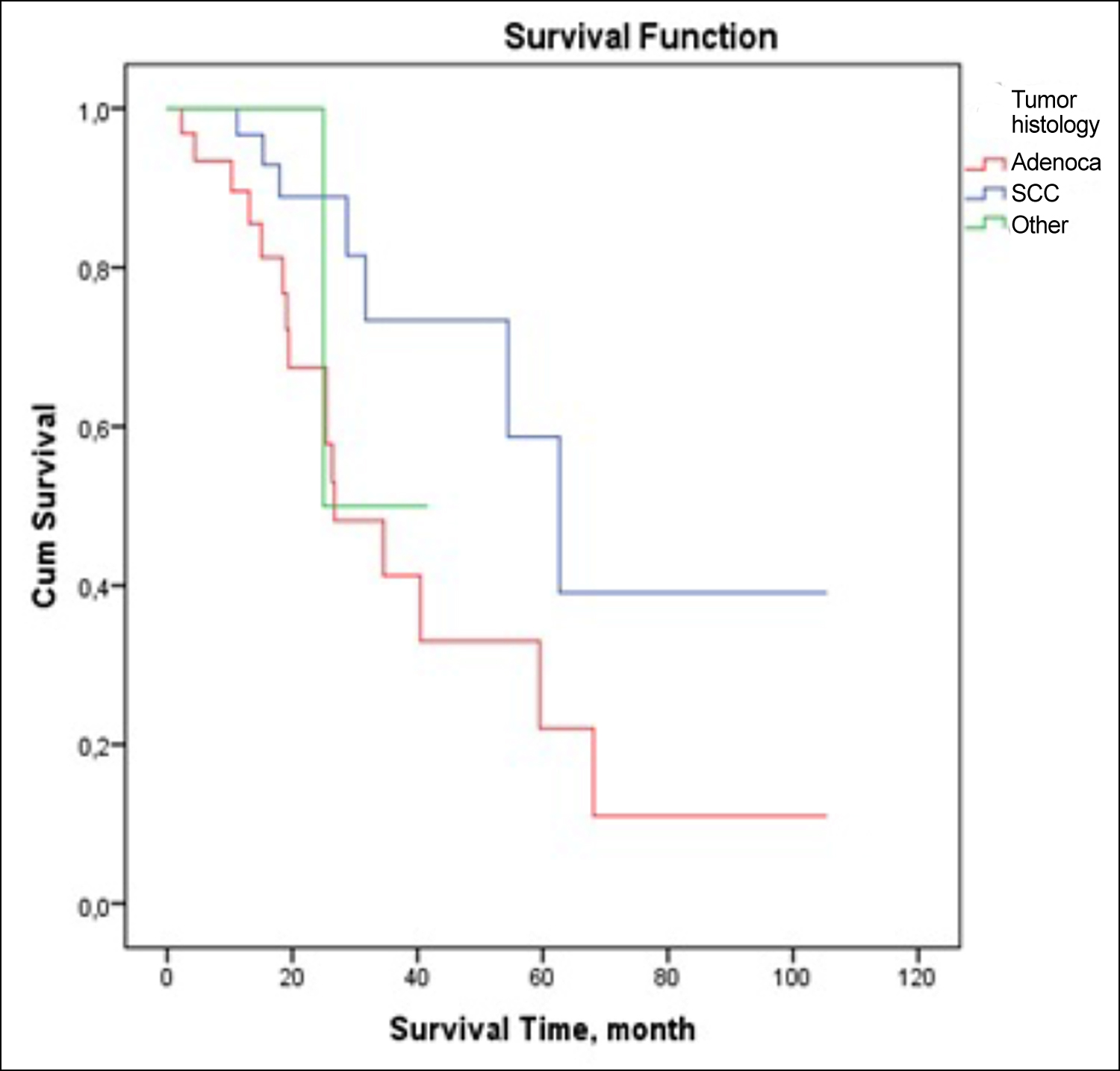 Figure 1: Distribution of tumor histology by survival time for recurrence or metastasis.
Figure 1: Distribution of tumor histology by survival time for recurrence or metastasis.
The 2-year survival rate was found to be 75.6%. A decrease in OS was observed in patients who developed recurrence (37.2 months) compared to patients who did not (87.6 months) (p=0.003, Figure 2). In patients who developed metastases, the OS was found to be 30.8 months, and it was found to be significantly lower than others (75.5 months) (p=0.001).
It was revealed that the overall survival of stage 1 patients was longer than stage 2 and 3 (p=0.046, Figure 2). It was observed that men who developed metastases lived longer than women (73.6 vs. 11 months p=0.011). Survival was numerically lower in patients with visceral pleura invasion, PNI, LVI, and >3 lymph nodes, but no statistically significant difference was determined. In the linear regression analysis, the factors that most affected survival was the development of metastasis and advanced stage, respectively (p<0.014, and p=0.022).
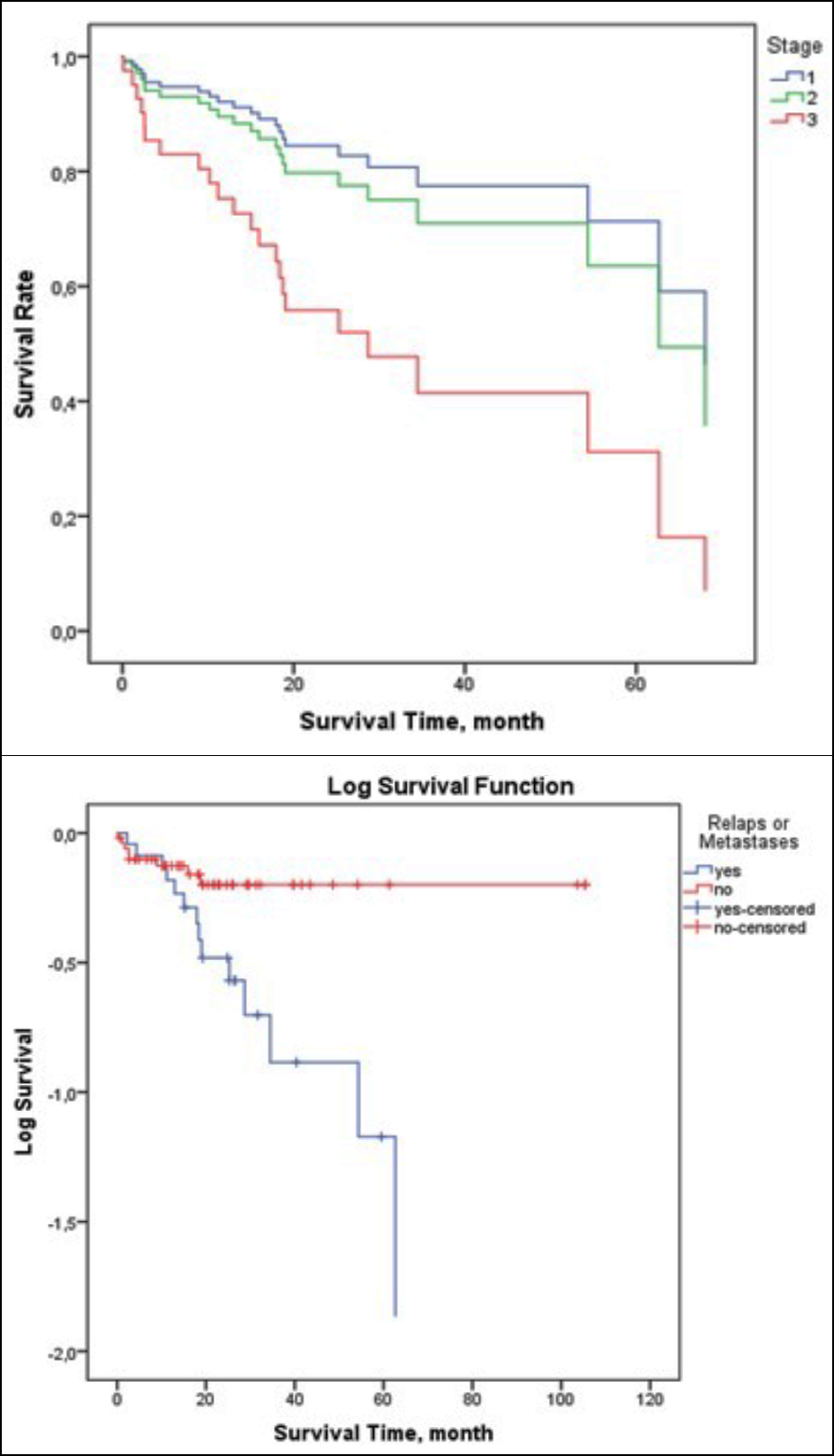 Figure 2: (A) Distribution of survival time by stage (B) Distribution of recurrence or metastasis by overall survival time.
Figure 2: (A) Distribution of survival time by stage (B) Distribution of recurrence or metastasis by overall survival time.
Prognostic factors of the disease were analysed using Cox-regression analyses. High MTV (p=0.014, and p=0.01) and TLG (p=0.022; and p=0.015) values of primary tumor on preoperative FDG-PET/CT, presence of Grade 2 disease (p<0.001; and p=0.001), development of recurrence (p=0.001; and p=0.001) were determined as factors affecting the prognosis negatively. Parameters that independently affect OS in the COX regression analysis are shown in Table III.
DISCUSSION
Lung cancer is the leading cause of cancer-related deaths in the world, and the 5-year survival rate is below 20% when all stages are taken into account.8 FDG-PET/CT, which has been used in the diagnosis, staging, and treatment response evaluation in lung cancer in recent years, provides information about tissue metabolism and standard cross-sectional methods.1 MTV and TLG parameters obtained in FDG-PET/CT scans show metabolic tumor volume and tumor metabolic activity in three dimensions.6 The contribution of metabolic parameters of FDG-PET/CT to estimate prognosis in various cancer types has been investigated. SUVmax is associated with tumor size in many studies, and it is a poor prognostic marker in some studies.5,9,10 MTV, and TLG are prognostic parameters that predict survival better than SUVmax.11 A meta-analysis conducted in 2016 showed that high SUVmax, MTV and TLG values estimated the risk of relapse or death5. The conflicting results on the SUVmax parameter can be attributed to the fact that it is affected by many factors (patient’s weight, plasma glucose level, patient preparation).12
Tumor size is one of the factors affecting prognosis. It was thought that metabolic tumor parameters would increase with high tumor diameter and predict survival, but this was shown in some studies but not others.13,14 This study also showed a relationship between metabolic parameters of FDG-PET/CT and increased tumor diameter. As the tumor volume increases, MTV and TLG will inevitably increase if necrosis has not developed. In addition, it has been reported that there are different metabolic involvement among histological types of the tumor.6,13,15 In this study, no relationship was found between histological subtypes and metabolic parameters, but it was found that patients with adenocarcinoma had a more aggressive course. Aggressive course due to histology has also been observed in some studies in the literature.16 It is known that metabolic parameters of FDG-PET/CT increase as the stage increases.17 It has been shown that level of metabolic parameters of FDG-PET/CT was increased, especially in the T stage. In these patients with 40.3% T1 and 34.8% lymph node involvement, it is thought that the N stage may have determined the stage, and as a result, there is no relationship between the level of metabolic parameters of FDG-PET/CT and the stage of the disease.
The prognostic contribution of MTV has been demonstrated in many studies.7,18 Even in early-stage patients, 18F-FDG-PET/CT is important in terms of identifying the high-risk patient group.5 TLG, which is considered the ideal parameter of viable tumor metabolism, is an independent prognostic factor for both OS and progression-free survival in patients with NSCLC.19 High TLG was related with decreased OS in patients with operated lung adenocarcinoma.6 High MTV and TLG values negatively affect survival regardless of histology, grade, stage, and operation type in this study.
It has been shown that metabolic parameters of FDG-PET/CT can also be used to evaluate treatment response in patients receiving chemotherapy and radiotherapy.20 Lung cancer is a heterogeneous group, and it is thought that many biological, molecular, and clinical factors affect the course of the tumor. As a result of advances in technology and treatments (immunotherapy and targeted therapies) in the future, it is hoped that patients with lung cancer will be caught early, high-risk groups will be identified, and survival will be extended with effective treatments.
The limited number of clients and the brief follow-up period were the factors limiting our study. Our advantages were that the study was conducted in a single-center, patient preparation stage before FDG-PET/CT, waiting time and FDG doses were standard, each patient was taken with the same FDG-PET/CT device, and a single nuclear medicine specialist re-evaluated all patients after the study was designed.
CONCLUSION
In early-stage NSCLC patients, MTV and TLG values, which can predict clinical course, recurrence, and even prognosis, can be obtained with FDG-PET/CT scanning while performing initial staging. Indication of these metabolic parameters, which can be obtained in all FDG-PET/CT examinations without extra cost and effort, will guide the clinician in post-operative patient management. Thus, it is hoped to prolong survival with close follow-up and effective treatments by identifying high-risk patients even at diagnosis.
PATIENTS’ CONSENT:
Since our study was a retrospective cohort study, patient consent was not obtained. Data usage permission and ethics committee application approval were obtained from the center where the study was conducted.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
EK, KT: Conception and design of work, obtaining data of patients who were operated on, the analysis and interpretation of, data, revision of work, drafting the article and revising it critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
SKG: Conception and design of work, nuclear medicine evaluations, measurements were done, the analysis and interpretation of data, drafting the article and revising it critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
REFERENCES
- Postmus PE, Kerr KM, Oudkerk M, Senan S, Waller DA, Vansteenkiste J, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017; 28:1-21. doi: 10.1093/annonc/mdx222.
- Chansky K, Sculier JP, Crowley J, Giroux DJ, Van Meerbeeck J, Goldstraw P. The IASLC lung cancer staging project: Prognostic factors and pathologic TNM stage in surgically managed non-small cell lung cancer. J Thorac Oncol 2009; 4(7):792-801. doi: 10.1097/JTO.0b013e3181a7716e.
- Volm M, Koomagi R. Relevance of proliferative and pro-apoptotic factors in nonsmall-cell lung cancer for patient survival. Br J Cancer 2000; 82:1747-17. doi: 10.1054/bjoc. 1999.1210.
- UyBico SJ, Wu CC, Suh RD, Le NH, Brown K, Krishnam MS. Lung cancer staging essentials: the new TNM staging system and potential imaging pitfalls. Radiographics 2010; 30:1163-1181. doi: 10.1148/rg.305095166.
- Liu J, Dong M, Sun X, Li W, Xing L, Yu J. Prognostic value of 18F-FDG PET/CT in surgical non-small cell lung cancer: A meta-analysis. PloS One 2016; 11(1):e0146195. doi: 10.1371/journal.pone.0146195.
- Ventura L, Scarlattei M, Gnetti L, Silini EM, Rossi M, Tiseo M, et al. Prognostic value of [18F] FDG PET/CT parameters in surgically resected primary lung adenocarcinoma: A single-center experience. Tumori J 2020; 106(3):212-22. doi: 10.1177/0300891620904404.
- Im HJ, Pak K, Cheon GJ, Kang KW, Kim SJ, Kim IJ, et al. Prognostic value of volumetric parameters of (18)F-FDG PET in non-small-cell lung cancer: A meta-analysis. Eur J Nucl Med Mol Imaging 2015; 42:241-51. doi: 10.1007/s00259- 014-2903-7.
- Howlader NN, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, et al. SEER cancer statistics review, 1975-2012, National Cancer Institute. Bethesda, MD.
- Ozgul MA, Kirkil G, Seyhan EC, Çetinkaya E, Ozgul G, Yuksel M. The maximum standardised FDG uptake on PET-CT in patients with non-small cell lung cancer. Multidisciplinary Respiratory Med 2013; 8(1):1-4. doi: 10.1186/2049- 6958-8-69.
- Berghmans T, Dusart M, Paesmans M, Hossein-Foucher C, Buvat I, Castaigne C, et al. Primary tumor standardised uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): A systematic review and meta-analysis (MA) by the European lung cancer working party for the IASLC lung cancer staging project. J Thoracic Oncol 2008; 3(1):6-12. doi: 10.1097/JTO.0b013e31815e6d6b.
- Davison J, Mercier G, Russo G, Subramaniam RM. PET-based primary tumor volumetric parameters and survival of patients with non — small cell lung carcinoma. Am J Roentgenol 2013; 200(3):635-40. doi: 10.2214/AJR.12. 9138.
- Lee P, Bazan JG, Lavori PW, Weerasuriya DK, Quon A, Le QT, et al. Metabolic tumor volume is an independent prognostic factor in patients treated definitively for non–small-cell lung cancer. Clin Lung Cancer 2012; 13(1):52-8. doi: 10.1016/j.cllc.2011.05.001.
- Downey RJ, Akhurst T, Gonen M, Vincent A, Bains MS, Larson S, et al. Preoperative F-18 fluorodeoxyglucose-positron emission tomography maximal standardised uptake value predicts survival after lung cancer resection. J Clin Oncol 2004; 22(16):3255-60. doi: 10.1200/JCO.2004. 11.109.
- Kurtipek E, Çayc M, Duzgun N, Esme H, Terzi Y, Bakdk S, et al. 18F-FDG PET/CT mean SUV and metabolic tumor volume for mean survival time in non–small cell lung cancer. Clin Nuclear Med 2015; 40(6):459-63. doi: 10.1097/RLU.0000 000000000740.
- Aquino SL, Halpern EF, Kuester LB, Fischman AJ. FDG-PET and CT features of non-small cell lung cancer based on tumor type. Int J Molecular Med 2007; 19(3):495-9.
- Russell PA, Wainer Z, Wright GM, Daniels M, Conron M, Williams RA. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new ınternational association for the study of lung cancer/American thoracic society/European respiratory society international multidisciplinary lung adenocarcinoma classification. J Thoracic Oncol 2011; 6(9):1496-504. doi: 10.1097/JTO.0b013e318221f701.
- Erol M. The relatıonshıp between 18 Fdg Pet/Ct parameters and clınıcal stage ın patıents wıth lung cancer. Selcuk Med J 2021; 37(1):24-31. doi:10.30733/std.2020.01475.
- Pellegrino S, Fonti R, Pulcrano A, Del Vecchio S. PET-based volumetric biomarkers for risk stratification of non-small cell lung cancer patients. Diagnostics 2021; 11(2):210. doi: 10.3390/diagnostics11020210.
- Vanhove K, Mesotten L, Heylen M, Derwael R, Louis E, Adriaensens P, et al. Prognostic value of total lesion glycolysis and metabolic active tumor volume in non-small cell lung cancer. Cancer Treat Res Commun 2018; 15:7-12. doi: 10.1016/j.ctarc.2017.11.005.
- Li L, Wang W, Campbell J, Waller JL, Piert M, Gross M, et al. Greater reduction in mid-treatment FDG-PET volume may be associated with worse survival in non-small cell lung cancer. Radiotherapy Oncol 2019; 132:241-9. doi: 10.1016/j.radonc.2018.10.006.