Complication Rates in Different Gastrectomy Techniques of Enhanced Recovery after Surgery for Gastric Cancer: A Meta-analysis
By Zhiming Chen1, Hui Xue2, Hua Yuan1, Jia Wang1, Qiuchen Wang1, Xiuying Zhang1Affiliations
doi: 10.29271/jcpsp.2022.10.1318Abstract
The purpose of this study was to analyse the postoperative complications of different gastrectomy methods, and provides guidance for the development of enhanced recovery after surgery (ERAS) protocols. We searched EMBASE, Web of Science, CINAHL, PubMed, MEDLINE, and the Cochrane Central Register of Controlled Trials for articles published from database inception to January 30, 2020. Statistical analysis was performed using R version 3.6.3 with single-rate meta-analysis. A total of 22 studies with 2127 patients were included. The types of postoperative complications showed that the pooled rate of nausea and vomiting was 10.22% (95% CI 4.56 to 17.48) and the pancreatic fistula was 3.58% (2.12 to 5.35) often occurred in patients who underwent laparoscopic gastrectomy (LG). After open gastrectomy (OG), postoperative urinary retention was 3.88% (0.00 to 13.17) and pancreatic fistula was 3.81% (1.78 to 6.42). The main complications of laparoscopic-assisted total gastrectomy and laparoscopic-assisted subtotal gastrectomy were pneumonia and pancreatic fistula, the rate was 3.19% (0.94 to 0.637) and 3.06% (0.11 to 8.36), respectively. In order to reduce the incidence of complications, ERAS should be revised from the aspects of rehabilitation, intraoperative application of new technology, shortening the operation time, early detection of high-risk groups, and implementation of audit.
Key Words: Enhanced recovery after surgery, Gastric cancer, Postoperative complications.
INTRODUCTION
The enhanced recovery after surgery (ERAS) protocol, a clinical multidisciplinary approach, is widely applied in gastrointestinal surgeries to expedite recovery, alleviate surgical stress, and reduce complication rates. However, ERAS protocol in gastric cancer seems to be unsatisfactory. For example, a recent meta-analysis showed that compared with conventional care,1-5 the ERAS protocol administered after gastric cancer surgery decreased length of hospital stay, costs, surgical stress response, and time for gut function to return but increased the risk of readmission.2,4,5 Postoperative complications have been repeatedly confirmed to affect the survival of patients and are also an important medical burden for healthcare providers.6-9
At present, ERAS has greatly shortened hospitalisation and accelerated functional recovery, and further development of prevention and treatment measures for complications may be the new development direction of ERAS.
Some researchers have confirmed that the items in the ERAS protocol should be modified based on the analysis of surgical complications. For example, through the analysis of the postoperative complications in the ERAS protocol for pancreatoduodenectomy, the existing problems of preoperative biliary drainage were determined and corresponding solutions were developed.10 The management of anaemia and other items in the ERAS protocol guidelines for colon cancer were also determined and adjusted by analysing surgical complications.11 Therefore, the analysis of complications is conducive to the optimisation of the ERAS protocol.
Existing gastrectomy procedures mainly include OG and LG. A nationwide study in Japan highlighted differences in the complications between OG and LG.12 This prompted us to speculate that the ERAS protocol in gastric cancer may be further optimised based on different complications of different surgical methods. Therefore, it is necessary to perform separate statistical analyses on the complications of different surgical methods. Due to the limitation of the number of samples in a single study, the clinical study of ERAS protocols for gastric cancer cannot provide comprehensive information on postoperative complications, so this meta-analysis was conducted to analyse postoperative complication rates of different gastrectomy techniques in patients with gastric cancer, to provide a reference for the optimisation of ERAS protocols.
METHODOLOGY
An electronic literature search of EMBASE, Web of Science, CINAHL, PubMed, and the Cochrane Central Register of Controlled Trials was conducted. It included all articles published from database inception to January 30, 2020. A systemic search was performed with the search terms "Stomach Neoplasms", "Gastroenterostomy", "Complication", and "enhanced recovery" in combination with the Boolean operators. The search strategy is shown in the supplementary Table I. The review was registered in the PROSPERO database (https://www.crd.york.ac.uk/PROSPERO) as record number CRD42020216400.
The results of systematic searches were imported into the reference manager (Endnote x9), and duplications were removed. Then, the titles and abstracts were reviewed to determine their relevance, and full-text articles were obtained for all studies that met the eligibility criteria. Two reviewers (CZM and WQC) conducted the study identification, and they used standardised methods for independent review according to the inclusion criteria below. The appropriate authors were contacted to collect missing data and assess study eligibility. Any differences in the assessment of study qualification were settled through mutual discussion or consultation with a third reviewer (HY).
The inclusion criteria were diagnosis of gastric cancer, age ≥18 years, ERAS after gastrectomy for gastric cancer, and reported postoperative complications of OG or LG. The different types of complications reported included any postoperative complications and were not limited to major complications during hospitalisation. Follow-up≤1 month was considered to investigate postoperative complications related to different operation methods, the time of occurrence of the complications related to operation was approximately one month.13 In order to expand the sample size, retrospective and prospective observational studies were included.
The exclusion criteria were studies that did not report the incidence of complications of gastrectomy for gastric cancer; postoperative follow-up periods of more than 1 month; participants received neoadjuvant chemotherapy or robot-assisted gastrectomy; carcinoma of the gastric stump combined with other malignant tumours; and fewer than six ERAS protocol items. Previous included studies containing 6-8 ERAS items.1,14 In order to expand the sample size, this study included studies containing more than 6 items.
Two authors independently extracted the information from the included studies and entered it into a standardised data collection form. For each study, the following characteristics were collected: author name, year of publication, study location, study design, number of patients, the incidence of complications, surgical methods, follow-up time and ERAS protocol items.
Total gastrectomy (TG) was defined as total resection of the stomach including the cardia and pylorus. This included laparoscopically assisted total gastrectomy (LATG) and open total gastrectomy (OTG). Subtotal gastrectomy (SG) was defined as stomach resection including the pylorus, with preserved cardia. In the standard gastrectomy, two-thirds of the stomach is resected. These included laparoscopically assisted subtotal gastrectomy (LASG), open subtotal gastrectomy (OSG), OG, and LG.15
The methodological quality of all studies was determined by two reviewers (QC W and ZM C) using the Newcastle-Ottawa scale for independent evaluation. Since this review only included the control group of randomised controlled studies, the quality of all randomised studies was assessed using criteria similar to those of prospective cohort studies. The maximum score for each study was 9 points, with higher scores indicating higher quality research.
R version 3.6.3 is used to perform single-rate meta-analysis. As a result of the low incidence of complications, the authors used Freeman Tukey’s double arcsine transformation.16 If the heterogeneity was significant, a random-effects model was adopted; otherwise, a fixed-effects model was adopted. Q and I2 values were used to assess heterogeneity. When p was 0.05, values of I2 ranging from 0% to 25% indicated low heterogeneity, from 25% to 75% indicated moderate heterogeneity, and above 75% indicated high heterogeneity.17
RESULTS
There were 417 studies identified by the literature search; 284 remained after duplications were removed by Endnote X9, 69 studies were included after a review of the titles and abstracts, and 22 studies were finally included after reading the full text.18-39 Twenty studies were identified for LG,18-35,38,39 and 8 studies were included for OG,18,20,22,23,28,36,37,39 Flow diagram of studies through the selection process are shown in Figure 1.
The specific characteristics of the included studies are shown in Table I. The studies were completed from 2010 to 2019. The 22 studies covered Asia, North America and Europe, of which 19 were performed in Asia. The main types of research included prospective observational studies, retrospective observational studies, prospective cohort studies, retrospective cohort studies, and randomised controlled trials. A total of 2,127 (22-403) patients were enrolled across the 22 studies. The median patient age across the studies ranged from 43 to 69 years. The number of complications reported in the included studies ranged from 2 to 13, including a total of 19 types of complications. The types of complications were reported in each study (Table I). A total of 16 ERAS items were in the 22 studies. All the included studies described more than 6 ERAS items, with an average of 10 (6-12) ERAS items in each study. Only two studies described no preanaesthetic medication in the ERAS protocol; all included postoperative analgesia and early oral feeding.
Table I: Characteristics of studies included in systematic review.
|
Study |
Year |
Setting |
Study design |
No. of patients |
Age (years) median (range) LG, OG |
Follow up period.(day) |
No. of complication |
Complicatins |
Quality score |
|
Abdikarim [19] |
2015 |
China |
RCT |
30 |
62 |
30 |
1 |
1,2,3,4,5,14 |
6 |
|
Sahoo [21] |
2014 |
India |
POS |
22 |
67(38-75) |
30 |
3 |
1,3,5,9,12,14,18 |
5 |
|
Liu [22] |
2016 |
China |
RCT |
84 |
69,68 |
_ |
11 |
1,3,6,7,8,9,10 |
6 |
|
Grantcharov [23] |
2010 |
Canada |
POS |
26 |
67 (40–86) |
30 |
4 |
1,2,5,7,8,10,11,12,16 |
7 |
|
Pedziwiatr [24] |
2014 |
Poland |
POS |
28 |
64( 39–86) |
_ |
1 |
4,8 |
5 |
|
Wong-Chong [25] |
2016 |
Canada |
PCS |
86 |
68 (28-85) |
30 |
10 |
1,2,3,7,9,11,12,14,16,19 |
7 |
|
Wu [26] |
2017 |
China |
POS |
41 |
63 |
_ |
10 |
1,3,7,9,10,14 |
7 |
|
Nakagawa [27] |
2018 |
Japan |
POS |
403 |
66(29–92) |
30 |
68 |
1,2,3,4,5,8,11,12,14,15,16,17,18 |
7 |
|
Liu [28] |
2016 |
China |
RCS |
525 |
56,57 |
30 |
20 |
1,2,7,14,16 |
4 |
|
Wang [29] |
2019 |
China |
RCT |
51 |
53 |
30 |
9 |
1,2,3,7,9,13,14,16 |
8 |
|
Mingjie [30] |
2017 |
China |
RCT |
73 |
61 (40–75) |
30 |
2 |
1,2,3,4,14 |
6 |
|
Zhang [31] |
2018 |
China |
RCT |
35 |
43 |
_ |
4 |
1,3,9,14 |
6 |
|
Zhou [32] |
2017 |
China |
RCS |
30 |
61 |
30 |
1 |
1,2,7,9,10,13,14 |
5 |
|
Aoyama [20] |
2014 |
Japan |
POS |
26 |
67 (45–76) 62 (38–79) |
30 |
2 |
3,4,5,9,14,16,18 |
7 |
|
Kim [33] |
2012 |
Korea |
RCT |
22 |
53 |
_ |
3 |
1,2,4,10 |
7 |
|
Lin [34] |
2019 |
China |
POS |
30 |
51 |
30 |
5 |
2,3,16,17 |
5 |
|
Xu [35] |
2017 |
China |
RCT |
30 |
60 |
30 |
6 |
2,3,6,7,12,14 |
4 |
|
Aoyama [18] |
2019 |
Japan |
RCT |
81 |
67 (36–80) 63 (33–79) |
30 |
9 |
1,2,3,4,5,7,9,14,16 |
6 |
|
Feng [36] |
2013 |
China |
RCT |
57 |
55 |
30 |
6 |
1,3,7,9,10,12,14,16 |
7 |
|
Lee [37] |
2014 |
Korea |
POS |
99 |
59 |
30 |
13 |
1,2,3,7,9,11,12,14,16 |
8 |
|
Wang [38] |
2010 |
China |
RCT |
45 |
59 |
30 |
9 |
1,2,3,6,8,10 |
7 |
|
Aoyama [39] |
2018 |
Japan |
ROS |
303 |
69(31-86) |
30 |
72 |
1,2,3,4,5,9,14,16 |
6 |
|
Abbreviations: POS,Prospective observational study ROS,Retrospective observational study PCS,Prospective cohort study RCS,Retrospective cohort study RCT,Randomised controlled trial;1 Wound infection;2 Intra-abdominal bleeding;3 Ileus;4 Anastomotic stenosis;5 Pancreatic fistula;6 Postoperative nausea and vomiting;7 Delayed gastric emptying;8 Urinary retention;9 Pneumonia;10 Urinary tract infection;11 Atelectasis;12 Deep venous thrombosis;13 Duodenal stump fistula;14 Anastomotic leakage;15 Ascites;16 Intra-abdominal abscess;17 Lymphorrhea;18 cardiocerebral vascular diseases;19 Sepsis. |
|||||||||
Table II: Comparison the odds ratio (OR) of complications between OG and LG.
|
Complications |
ORa |
[95% Conf. interval] |
p-value |
I2 |
|
Wound infection |
0.528 |
[0.154,1.804] |
0.308 |
0% |
|
Intra-abdominal bleeding |
1.020 |
[0.211,4.921] |
0.981 |
0% |
|
Ileus |
1.236 |
[0.405,3.771] |
0.710 |
0% |
|
Anastomotic stenosis |
1.264 |
[0.260,5.962] |
0.767 |
0% |
|
Pancreatic fistula |
1.245 |
[0.513,3.019] |
0.628 |
0% |
|
Delayed gastric emptying |
0.513 |
[0.093,2.837] |
0.444 |
62% |
|
Urinary retention |
0.408 |
[0.098,1.704] |
0.219 |
0% |
|
Pneumonia |
1.726 |
[0.578,5.153] |
0.328 |
0% |
|
Urinary tract infection |
0.897 |
[0.125,6.455] |
0.884 |
0% |
|
Anastomotic leakage |
0.977 |
[0.425,2.247] |
0.956 |
20% |
|
Intra-abdominal abscess |
1.331 |
[0.401,4.413] |
0.640 |
0% |
|
a OG is reference |
||||
The quality of each study was assessed using the previously validated Newcastle-Ottawa scale, with a median (range) total score of 6 (4-8, Table I). Begg’s test (p=0.477) showed that the publication bias was not statistically significant.
Pooled complication rates for LG and OG were calculated across six studies with their respective odds ratios (OR, Table II).18,20,22,23,28,39 There was no significant difference in the incidence of postoperative complications between LG and OG. When all p-values were >0.05, the maximum value of I2 was 62 %, and the minimum value was 0.0%.
The difference in the complications was determined with the highest incidence by analysing twenty studies that reported postoperative complications in patients undergoing LG under an ERAS protocol,18-35,38,39 and in 8 that reported complications for patients undergoing OG.18,20,22,23,28,36,37,39 The complication rate of OG were higher than that of LG in wound infection (OG 0.24% (95% CI 0.00 to 1.02); LG 0.21% (0.00 to 0.72)), pancreatic fistula (OG 3.81% (1.78 to 6.42); LG 3.58% (2.12 to 5.35)), urinary retention (OG 3.88% (0.00 to 13.17); LG 0.93% (0.00 to 5.12)), deep venous thrombosis (OG 0.07% (0.00 to 2.12); 0.00% (0.00 to 0.50)) and anastomotic leakage (OG 1.72% (0.69 to 3.05); LG 0.81% (0.02 to 2.25)). The most common complication for OG was urinary retention (3.88%, 0.00 to 13.17), followed by pancreatic fistula (3.81%, 1.78 to 6.42) and anastomotic leakage (1.72%, 0.69 to 3.05). Postoperative nausea and vomiting (PONV) (10.22%, 4.56 to 17.48) were the most common complication after LG, followed by pancreatic fistula (3.58%) and pneumonia (2.75%), as presented in Figure 2. Among the studies reporting complications of LG, those describing delayed gastric emptying (I2=68%, p=0.001), urinary retention (I2=61%, p=0.05), atelectasis (I2=84%, p=0.002) and anastomotic leakage (I2=52%, p=0.05) showed significant heterogeneity, and a random-effects model was used. Among the studies reporting complications of OG, those describing intra-abdominal bleeding (I2=63%, p=0.03), delayed gastric emptying (I2=71%, p=0.004), and pneumonia (I2=65%, p=0.01) showed significant heterogeneity, and a random-effects model was again used. The remaining studies showed low heterogeneity, and so a fixed-effects model was used.
Six studies involving 310 participants reported complications of LATG, with pneumonia having the highest incidence (3.19%, 0.94 to 6.37), followed by intra-abdominal abscesses (3.01%, 0.69 to 6.46). Only studies reporting anastomotic leakage (I2=55%, P=0.05) after LATG demonstrated significant heterogeneity. Due to the limited number of articles, we cannot analyse the postoperative complications of OTG and OSG.
A total of 7 studies (a total of 266 patients) reported complications after LASG, with the highest incidence reported for pancreatic fistula (3.06%, 0.11 to 8.36) followed by intra-abdominal abscesses (1.84%, 0.20 to 4.73) (Figure 3). There was no significant heterogeneity for the studies on LASG, with a maximum value of I2 of 58% (p >0.05). For high heterogeneity, a random effect model was used.
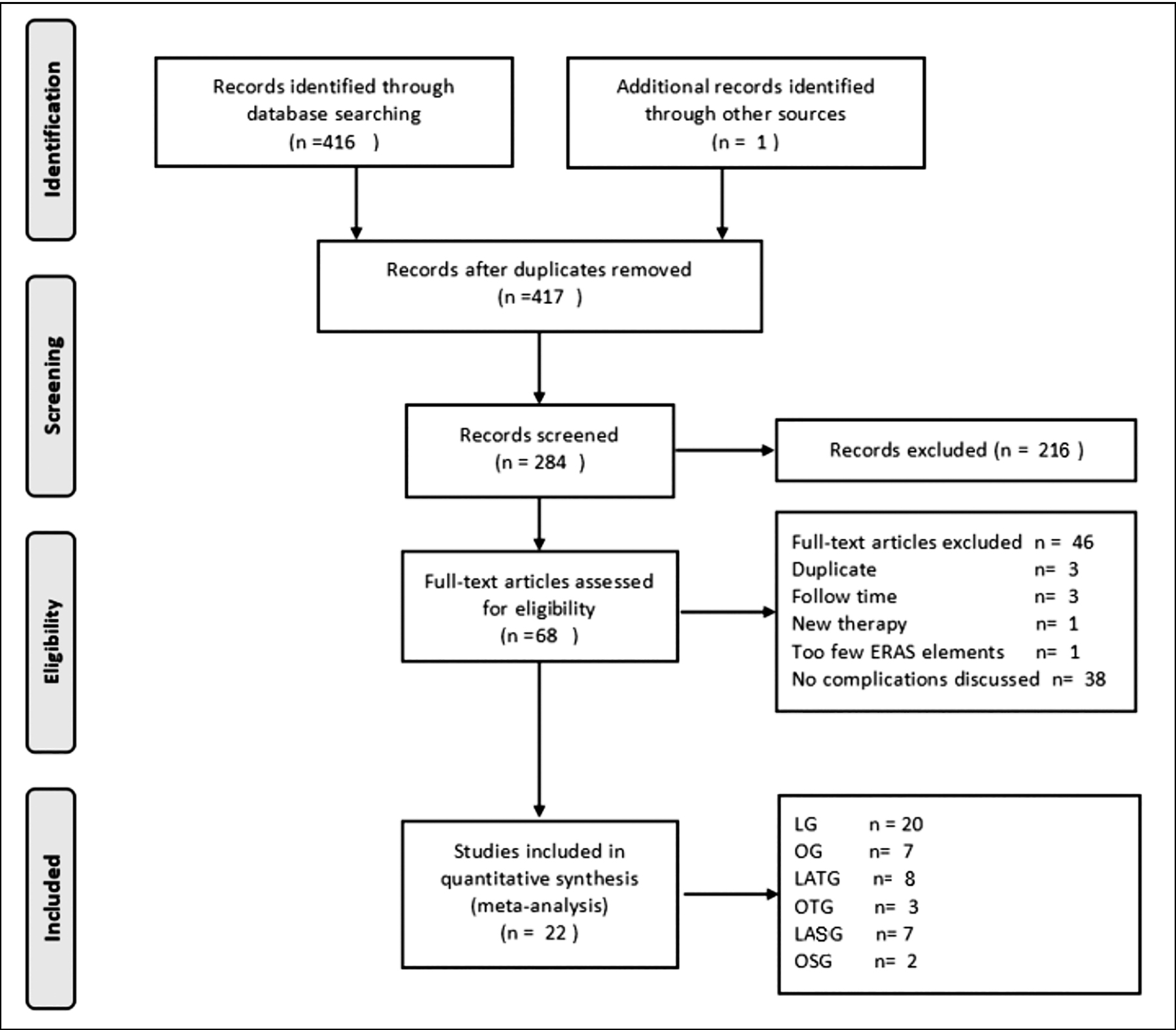 Figure 1: Flow diagram of systematic literature review.
Figure 1: Flow diagram of systematic literature review.
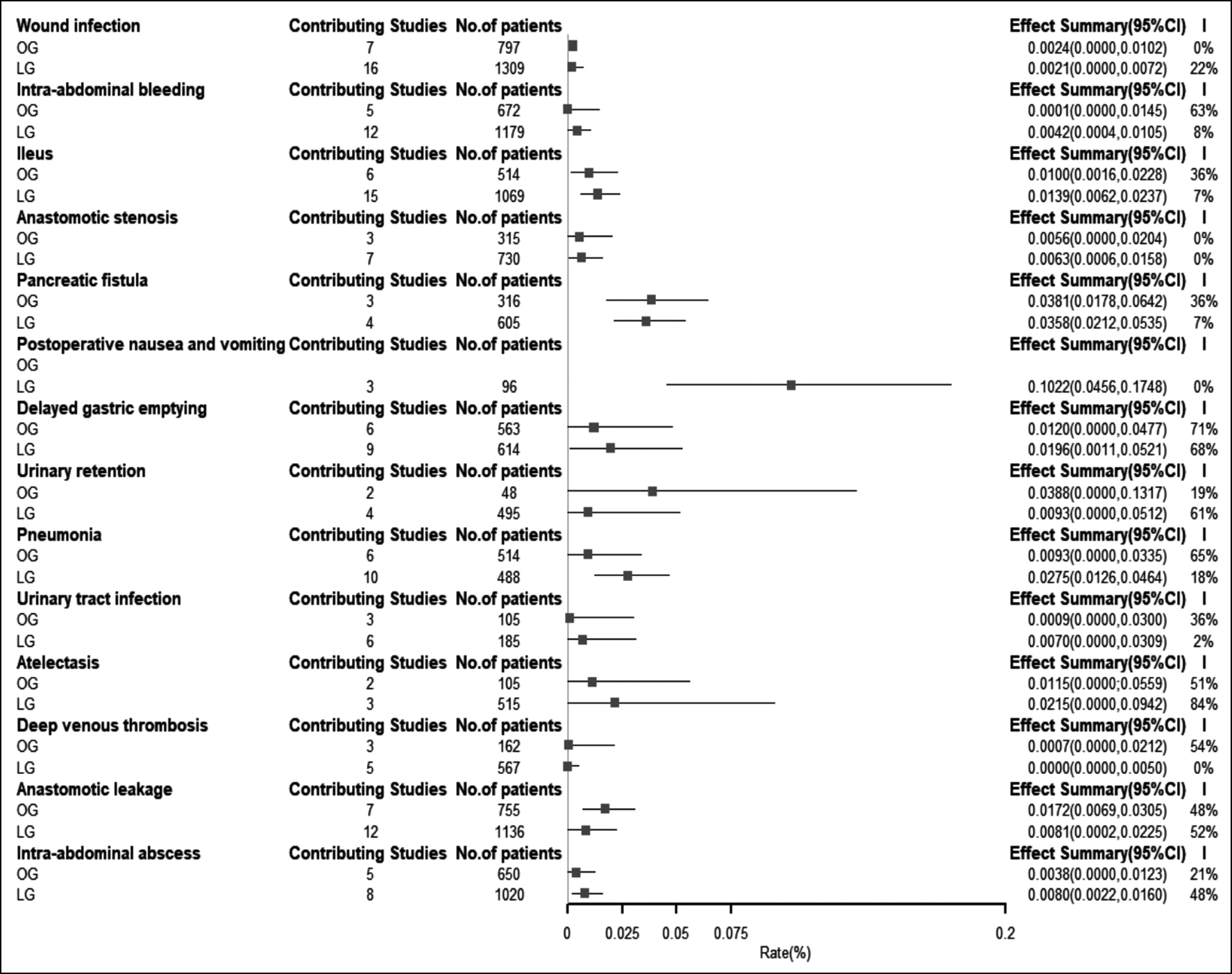 Figure 2: Forest plot of different complication rates of LG and OG.
Figure 2: Forest plot of different complication rates of LG and OG.
DISCUSSION
The extensive implementation of ERAS has greatly accelerated the functional recovery of patients and reduced the length of hospitalisation. Further research on ERAS should focus on improving the quality of patients' recovery, such as reducing complications and improving the quality of life. Researchers have suggested that the analysis of complications can guide the development of ERAS protocols.10,11 The present study conducted a meta-analysis of complication rates for LG and OG under the ERAS protocol to provide evidence for improving this protocol for gastric cancer. This study found that the ERAS protocol items included in the articles were inconsistent. The postoperative complication rates were not significantly different between OG and LG. However, the types and incidence of complications between OG and LG were different. Those patients who underwent LG mainly suffered from PONV (10.22%) and pancreatic fistula (3.58%). Patients who underwent OG mainly suffered from urinary retention (3.88%) and pancreatic fistula (3.81%). Patients who underwent LATG primarily suffered from pneumonia (3.19%) and intra-abdominal abscess (3.01%). The most common complication among patients who underwent LASG was pancreatic fistula (3.06%).
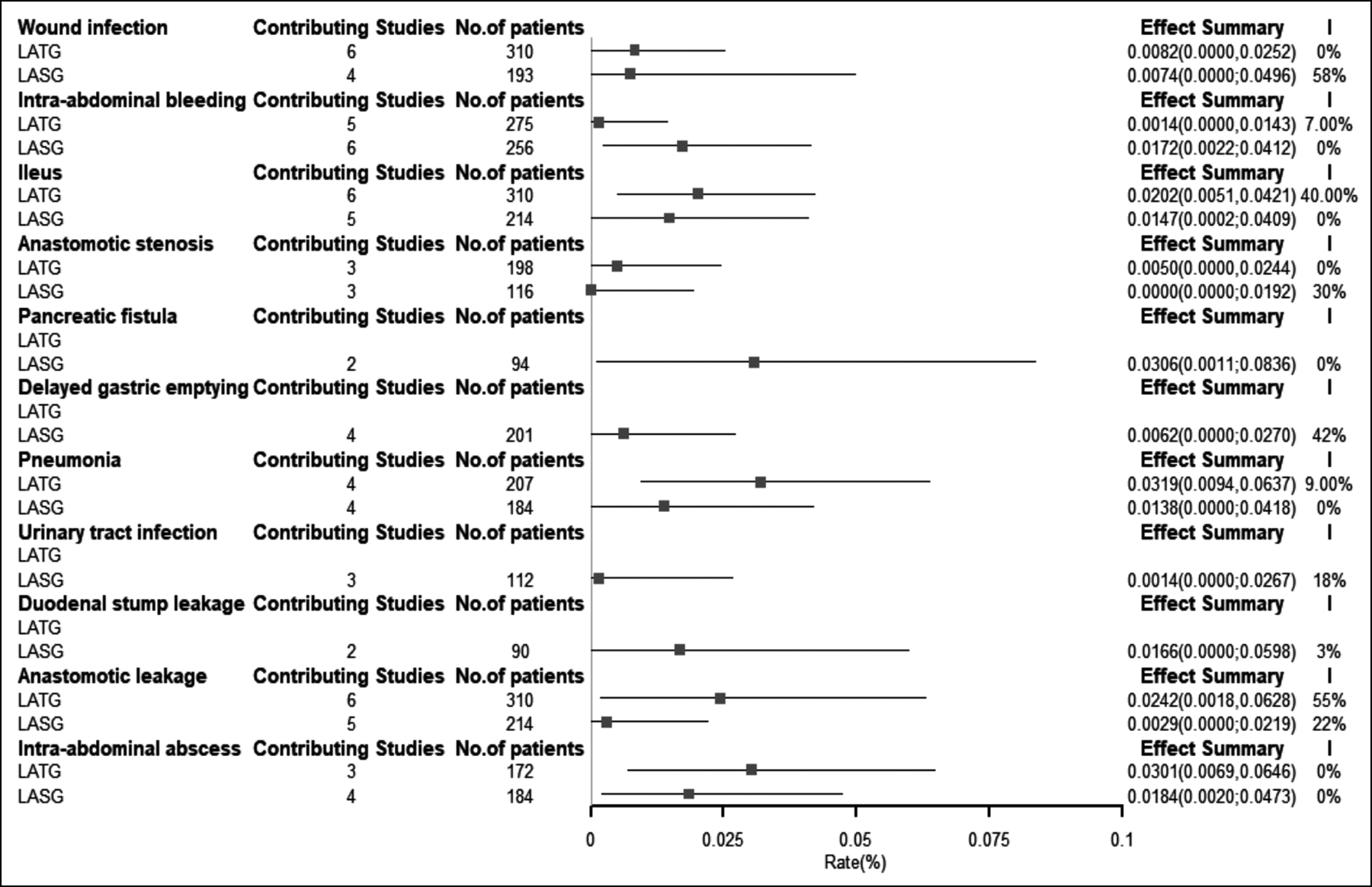 Figure 3: Forest plot different complication rates of LATG and LASG.
Figure 3: Forest plot different complication rates of LATG and LASG.
The frequency of PONV in LG groups was 10.22%, which is consistent with the latest PONV management guidelines showing laparoscopy is a risk factor for PONV.40 The multimodal approach to PONV prophylaxis is recommended for high-risk patients in the latest PONV management guidelines. The ondansetron and dexamethasone effectively reduced the incidence of nausea and vomiting induced by patient-controlled analgesia (PCA) after laparoscopic cholecystectomy.41,42 In addition, some studies regarded PONV as a symptom of ileus and delayed gastric emptying, so the complications were not recorded.27,38 Therefore, the actual incidence may be higher than that reported by the authors. Reducing the incidence of nausea and vomiting should be included in the ERAS items for LG patients, to improve the prevention and management awareness of healthcare workers.
These results indicate that the incidence of the pancreatic fistula was observed in 3.58%, 3.06%, and 3.81% of LG, LASG and OG patients, respectively, the comparison of the incidence of LG and OG showed no significant difference. Other studies have also shown a higher incidence of pancreatic fistula in LG (27.0%) than in OG (22.9%),12,43 without any significant difference. The reason for the difference may be due to the incidence of pancreatic fistula in OG from a small sample size and the average age of LG is older than OG in this study. Studies have shown that both OG and LG should avoid squeezing the pancreas during surgery, and LG should also pay attention to the late thermal damage of the pancreas from energy devices.43,44 At present, the measurement of amylase in the drainage fluid can predict severe postoperative pancreatic fistula after gastric cancer surgery.45 However, the ERAS protocol for gastric cancer surgery does not recommend postoperative abdominal drainage tubes because they may increase complications and prolong the length of hospital stay. The predictive factors of pancreatic fistula are also different in LG and OG.42 For LG patients who are older and have a low preoperative lymphocyte count, the position of the pancreas can predict the occurrence of postoperative pancreatic fistula.46 In addition to the identification of high-risk groups, the current studies also confirm that robotic surgery and new surgical methods can reduce the occurrence of complications.44,47 The ERAS protocol should identify population with a high-risk of pancreatic fistula based on different surgical procedures. Both OG and LG should avoid compression of the pancreas and adopt new techniques to reduce postoperative complications.
The incidence rate of urinary retention in OG groups was the highest, 3.88%, while in LG groups was 0.93%, the incidence rate varied greatly, the sample size was relatively small, and the generalisability of the results was, therefore, unclear. But some small sample studies of abdominal surgery also showed a higher incidence of urinary retention.48-50 Some studies have reported that patients fear abdominal pain after open surgery and try to avoid abdominal force, resulting in the reduction of abdominal pressure and diaphragmatic muscle movement, which may be the main causes of urinary retention.51,52 Therefore, better postoperative analgesia can reduce the patients’ abdominal pain, so as to reduce the occurrence of urinary retention. Epidural anaesthesia was recommended by ERAS protocol; studies showed that epidural anaesthesia was better than PCA in pain relief.52 The studies on urinary retention were included which OG patients used the epidural and intravenous infusion of analgesics, LG patients mostly adopted PCA, but OG incidence of urinary retention did not decrease. It is confirmed that epidural anaesthesia is an independent risk factor for urinary retention.53 Epidural anaesthesia reduces postoperative pain, but the cause of increasing urinary retention may be the wrong sequence of catheter withdrawal and stopping epidural anaesthesia.48 However, the ERAS protocol recommends that the catheter be removed within 24-48 hours after surgery, but the duration of epidural anaesthesia is more than two days. In conclusion, the optimal order and interval of removal of epidural and urethral catheters should be considered to avoid urinary retention in ERAS protocol.48,50
Respiratory complications were common in both LG and OG patients, which was consistent with Ushimaru et al.’s report.54 The incidence of LG (2.75%) pneumonia was higher than OG (0.93%). The possible reason is that the longer the operation time is, the more serious ventilator-induced lung injury.55 Further study has shown that intraoperative lung-protective mechanical ventilation can reduce ventilator-induced lung injury.55 Chinese 2018 ERAS protocol recommends that protective ventilation during operation should be applied.56 Intraoperative protective ventilation was not included by the ERAS protocol items of any article, while this study included. In addition, the implementation of a prehabilitation plan can improve the cardiopulmonary function of patients and reduce the incidence of postoperative respiratory complications.57 Prehabilitation has a positive impact on postoperative results in which the patient undergo abdominal surgery,58 and is also included in the ERAS protocol for colon cancer. Unfortunately, in the included studies, only one study on LG patients used a prehabilitation nutritional support program. Due to the small sample size, the reported incidence of pneumonia was 4.5%, which was higher than that of LG patients (2.75%).21 For OG, the authors can consider revising the ERAS protocol from both preoperative rehabilitation and intraoperative protective ventilation to reduce the incidence of pneumonia. On the basis of OG, ERAS should be revised from the perspective of reducing intraoperative time for LG patients.
Here, various factors are reviewed in the ERAS protocols, but the rate of complications does not decrease. ERAS protocol compliance is related to complications, and good compliance also reduces the rate of complications and the length of stay. Compliance can be divided into patient compliance and protocol compliance.59,60 However, most studies in this meta-analysis did not describe problems with ERAS protocol compliance. We could see from the number of protocol items that most of the studies only implemented parts of the ERAS protocols. The audit was absent in nearly all of the included studies. Audit forms the basis for insights to practice and outcomes. ERAS protocol should include an audit to increase compliance among ERAS protocol, that help to reduce complications. Recent consensus on training and implementation of ERAS also highlights the importance of audits.61 The physical condition of patients before surgery will also affect their complications.21,25
There are some limitations of this study. The follow-up time was not strictly controlled, and studies being performed within 30 days after discharge were included. Some studies only collected data before discharge, which results in some patients’ data not being collected. In order to expand the sample size, the type of research was not strictly limited; including some retrospective studies may have incomplete data. The included studies did not clearly define the diagnostic criteria of complications, so the diagnosis of complications was different in different studies, which may be the main source of heterogeneity. The main purpose of this paper is to analyse the frequency of complications and provide suggestions for ERAS protocol to prevent and reduce postoperative complications. Therefore, this paper did not collect or analyse the severity of complications.
CONCLUSION
It would be of great importance to develop ERAS in different gastrectomy methods for gastric cancer. The most common complication of OG was urinary retention, followed by pancreatic fistula. Postoperative nausea and vomiting were the most common complication after LG, followed by pancreatic fistula and pneumonia. ERAS protocol should consider the benefits of rehabilitation plan, operation time control, time and sequence of pipeline removal for patients. ERAS should also improve the detection of high-risk groups, early case identification and prompt management. Audit should be incorporated in the ERAS protocol.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
ZC: Contributed to the performance of the study, analysis and interpretation of the data, and drafted the manuscript.
HY: Contributed to the supervision of the study and interpreted of the data.
JW: Contributed to the analysis and interpretation of the data.
QW: Contributed to the performance of the study and revised the manuscript.
HX, XZ: Contributed to the conception of the study, performed the study, interpreted the data, and significantly revised the manuscript.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Li Z, Wang Q, Li B. Influence of enhanced recovery after surgery programs on laparoscopy-assisted gastrectomy for gastric cancer: A systematic review and meta-analysis of randomised control trials. World J Surg Oncol 2017; 15(1): doi: 10.1186/s12957-017-1271-8.
- Wee IJY, Syn NL, Shabbir A. Enhanced recovery versus conventional care in gastric cancer surgery: A meta-analysis of randomised and non-randomised controlled trials. Gastric cancer. Japanese Gastric Cancer Assoc 2019; 22(3):423-34. doi: 10.1007/s10120-019-00937-9.
- Yamagata Y, Yoshikawa T, Yura M. Current status of the "enhanced recovery after surgery" program in gastric cancer surgery. Ann Gastroenterological Surg 2019; 3(3):231-8. doi: 10.1002/ags3.12232.
- Lee Y, Yu J, Doumouras AG. Enhanced recovery after surgery (ERAS) versus standard recovery for elective gastric cancer surgery: A meta-analysis of randomised controlled trials. Surg Oncol 2020; 32:75-87. doi: 10.1016/ j.suronc.2019.11.004.
- Ding J, Sun B, Song P. The application of enhanced recovery after surgery (ERAS)/fast-track surgery in gastrectomy for gastric cancer: A systematic review and meta-analysis. Oncotarget 2017; 8(43):75699-711. doi: 10.18632/ oncotarget.18581.
- Wang S, Xu L, Wang Q. Postoperative complications and prognosis after radical gastrectomy for gastric cancer: A systematic review and meta-analysis of observational studies. World J Surg Oncol 2019; 17(1):52. doi: 10.1186/ s12957-019-1593-9.
- Khuri SF, Henderson WG, DePalma RG. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg 2005; 242(3):326-41; discussion 41-3. doi: 10.1097/01.sla.0000 179621.33268.83.
- Yuan P, Wu Z, Li Z. Impact of postoperative major complications on long-term survival after radical resection of gastric cancer. BMC Cancer 2019; 19(1):833. doi: 10.1186/ s12885-019-6024-3.
- Patel AS, Bergman A, Moore BW. The economic burden of complications occurring in major surgical procedures: A systematic review. Appl Health Econ Health Policy 2013; 11(6):577-92. doi: 10.1007/s40258-013-0060-y.
- Melloul E, Lassen K, Roulin D, Grass F, Perinel J, Adham M, et al. Guidelines for perioperative care for pancreato-duodenectomy: Enhanced recovery after surgery (ERAS) recommendations 2019. World J Surg 2020; 44(7):2056-84. doi: 10.1007/s00268-020-05462-w.
- Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N, et al. Guidelines for perioperative care in elective colorectal surgery: Enhanced recovery after surgery (ERAS(®) society recommendations: 2018. World J Surg 2019; 43(3):659-95. doi: 10.1007/s00268-018-4844-y.
- Hiki N, Honda M, Etoh T. Higher incidence of pancreatic fistula in laparoscopic gastrectomy. Real-world evidence from a nationwide prospective cohort study. Gastric cancer. Japanese Gastric Cancer Assoc 2018; 21(1):162-70. doi: 10.1007/s10120-017-0764-z.
- Baiocchi GL, Giacopuzzi S, Marrelli D. Complications after gastrectomy for cancer: Italian perspective. Updates Surg 2017; 69(3):285-88. doi: 10.1007/s13304-017-0478-0.
- Wang LH, Zhu RF, Gao C. Application of enhanced recovery after gastric cancer surgery: An updated meta-analysis. World J Gastroenterol 2018; 24(14):1562-78. doi: 10. 3748/wjg.v24.i14.1562.
- Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric cancer. Japanese Gastric Cancer Assoc 2017; 20(1):1-19. doi: 10.1007/s10120-016-0622-4.
- Barendregt JJ, Doi SA, Lee YY. Meta-analysis of prevalence. J Epidemiol Community Health 2013; 67(11):974-8. doi: 10.1136/jech-2013-203104.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics Medicine 2002; 21(11):1539-58. doi: 10.1002/sim.1186.
- Aoyama T, Yoshikawa T, Sato T. Equivalent feasibility and safety of perioperative care by ERAS in open and laparoscopy-assisted distal gastrectomy for gastric cancer: A single-institution ancillary study using the patient cohort enrolled in the JCOG0912 phase III trial. Japanese Gastric Cancer Assoc 2019; 22(3):617-23. doi: 10.1007/s10120- 018-0873-3.
- Abdikarim I, Cao XY, Li SZ. Enhanced recovery after surgery with laparoscopic radical gastrectomy for stomach carcinomas. World J Gastroentero 2015; 21(47):13339-44. doi: 10.3748/wjg.v21.i47.13339.
- Aoyama T, Yoshikawa T, Hayashi T. Randomised comparison of surgical stress and the nutritional status between laparoscopy-assisted and open distal gastrectomy for gastric cancer. Ann Surg Oncol 2014; 21(6):1983‐90. doi: 10.1245/s10434-014-3509-9.
- Sahoo MR, Gowda MS, Kumar TA. Early rehabilitation after surgery program versus conventional care during perioperative period in patients undergoing laparoscopic assisted total gastrectomy. J Minimal Access Surgery 2014; 10(3):132-8. doi: 10.4103/0972-9941.134876.
- Liu G, Jian F, Wang X. Fast-track surgery protocol in elderly patients undergoing laparoscopic radical gastrectomy for gastric cancer: A randomised controlled trial. Oncotargets Therapy 2016; 9:3345‐51. doi: 10.2147/OTT.S107443.
- Grantcharov TP, Kehlet H. Laparoscopic gastric surgery in an enhanced recovery programme. British J Surg 2010; 97(10):1547-51. doi: 10.1002/bjs.7184.
- Pedziwiatr M, Matlok M, Kisialeuski M. Short hospital stays after laparoscopic gastric surgery under an enhanced recovery after surgery (ERAS) pathway: Experience at a single center. European Surgery-Acta Chirurgica Austriaca 2014; 46(3):128-32. doi: 10.1007/s10353-014-0264-x.
- Wong-Chong N, Kehlet H, Grantcharov TP. Outcomes from an enhanced recovery program for laparoscopic gastric surgery. Surg Laparoscopy Endoscopy Percutaneous Techniques 2016; 26(3):50-5. doi: 10.1097/SLE.00000 00000000277.
- Wu JY, Sha HC, Ren XT. Fast-track surgery could improve postoperative recovery in patients with laparoscopy D2 Gastrectomy. Int Surg 2017; 102(3-4):151-6. doi: 10. 9738/intsurg-d-17-00110.1.
- Nakagawa M, Tomii C, Inokuchi M, Otsuki S, Kojima K. Feasibility of a clinical pathway with early oral intake and discharge for laparoscopic gastrectomy. Scand J Surg 2018; 107(3):218-23. doi: 10.1177/1457496917748228.
- Liu XX, Pan HF, Jiang ZW, Zhang S, Wang ZM, Chen P, et al. "Fast-track" and "minimally invasive" surgery for gastric cancer. Chin Med J (Engl) 2016; 129(19):2294-300. doi: 10.4103/0366-6999.190659.
- Wang Q, Yang KL, Guo BY. Safety of early oral feeding after total laparoscopic radical gastrectomy for gastric cancer (SOFTLY-1): A single-center randomised controlled trial. Cancer Manag Res 2019; 11:4839-46. doi: 10.2147/cmar. s199552.
- Mingjie X, Luyao Z, Ze T. Laparoscopic radical gastrectomy for resectable advanced gastric cancer within enhanced recovery programs: A prospective randomised controlled trial. J Laparoendoscopic Adv Surg Tech A 2017; 27(9): 959-64. doi: 10.1089/lap.2016.0057.
- Zhang XF, Zhong W. Effect of fast track surgery concept based nursing intervention on rehabilitation and nursing satisfaction in patients with advanced gastric cancer after laparoscopic assisted D2 radical operation. World Chinese J Digestol 2018; 26(5):325-31. doi: 10.11569/wcjd. v26.i5.325.
- Zhou JF, He QL, Wang JX. Application of enhanced recovery after surgery in single-incision laparoscopic distal gastrectomy. Surg Laparosc Endosc Percutan Tech 2017; 27(6):449-55. doi: 10.1097/sle.0000000000000474.
- Kim JW, Kim WS, Cheong JH. Safety and efficacy of fast-track surgery in laparoscopic distal gastrectomy for gastric cancer: A randomised clinical trial. World J Surg 2012; 36(12):2879-87. doi: 10.1007/s00268-012-1741-7.
- Lin T, Yu J, Hu Y, Liu H, Lu Y, Zhao M, et al. Preliminary experience of dual-port laparoscopic distal gastrectomy for gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi 2019; 22(1):35-42.
- Xu X, Xu J. Application of enhanced recovery after surgery in laparoscopy-assisted distal gastrectomy. Cancer Research Clinic 2017; 29(3):180-3. doi: 10.3760/cma.j.issn.1006- 9801.2017.03.009.
- Feng F, Ji G, Li JP. Fast-track surgery could improve postoperative recovery in radical total gastrectomy patients. World J Gastroenterol 2013; 19(23):3642-48. doi: 10.3748/ wjg.v19.i23.3642.
- Lee J, Jeon H. The clinical indication and feasibility of the enhanced recovery protocol for curative gastric cancer surgery: Analysis of 147 consecutive experiences. Digestive Surg 2014; 31(4-5):318-23. doi: 10.1159/000 368091.
- Wang D, Kong Y, Zhong B. Fast-track surgery improves postoperative recovery in patients with gastric cancer: A randomised comparison with conventional postoperative care. J Gastrointestinal Surg 2010; 14(4):620-7. doi: 10. 1007/s11605-009-1139-5.
- Aoyama T, Yoshikawa T, Maezawa Y. A Comparison of the body composition changes between laparoscopy-assisted and open total gastrectomy for gastric cancer. In vivo (Athens, Greece) 2018; 32(6):1513-8. doi: 10.21873/invivo. 11408.
- Gan TJ, Belani KG, Bergese S. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesthesia Analgesia 2020; 131(2):411-48. doi: 10.1213/ ane.0000000000004833.
- Li Y, Deng R, Zhou J. Comparison of ramosetron and ondansetron for the prevention of postoperative nausea and vomiting in patients undergoing laparoscopic surgery: A meta-analysis of randomised controlled trials. J Int Med Res 2019; 47(10):4591-603. doi: 10.1177/03000 60519871171.
- Sridharan K, Sivaramakrishnan G. Drugs for preventing post-operative nausea and vomiting in patients undergoing laparoscopic cholecystectomy: Network meta-analysis of randomised clinical trials and trial sequential analysis. Int J Surg (London, England) 2019; 69:1-12. doi: 10.1016/j.ijsu. 2019.07.002.
- Kinoshita J, Yamaguchi T, Saito H. Comparison of prognostic impact of anatomic location of the pancreas on postoperative pancreatic fistula in laparoscopic and open gastrectomy. BMC Gastroenterol 2020; 20(1):325. doi: 10.1186/s12876-020-01476-9.
- Tsujiura M, Hiki N, Ohashi M. "Pancreas-compressionless gastrectomy": A novel laparoscopic approach for suprapancreatic lymph node dissection. Ann Surgical Oncol 2017; 24(11):3331-7. doi: 10.1245/s10434-017-5974-4.
- Kamiya S, Hiki N, Kumagai K. Two-point measurement of amylase in drainage fluid predicts severe postoperative pancreatic fistula after gastric cancer surgery. Japanese Gastric Cancer Assoc 2018; 21(5):871-78. doi: 10.1007/ s10120-018-0805-2.
- Komatsu S, Ichikawa D, Kashimoto K. Risk factors to predict severe postoperative pancreatic fistula following gastrec-tomy for gastric cancer. World J Gastroenterol 2013; 19(46):8696-702. doi: 10.3748/wjg.v19.i46.8696.
- Guerrini GP, Esposito G, Magistri P. Robotic versus laparoscopic gastrectomy for gastric cancer: The largest meta-analysis. Int J Surg (London, England) 2020; 82: 210-8. doi: 10.1016/j.ijsu.2020.07.053.
- Hayami S, Ueno M, Kawai M. Optimal timing of removal of epidural and urethral catheters to avoid postoperative urinary retention undergoing abdominal surgery. Digestive Surg 2019; 36(3):261-5. doi: 10.1159/000490199.
- Papageorge CM, Howington B, Leverson G. Preoperative tamsulosin to prevent postoperative urinary retention: A randomised controlled trial. J Surg Res 2021; 262:130-39. doi: 10.1016/j.jss.2020.12.055.
- Yanagimoto Y, Takiguchi S, Miyazaki Y. Comparison of pain management after laparoscopic distal gastrectomy with and without epidural analgesia. Surg Today 2016; 46(2):229-34. doi: 10.1007/s00595-015-1162-y.
- Garg P. Inability to raise intraabdominal pressure (IRIP): A common missed cause of postoperative urinary retention after anorectal surgery. Techniques Coloproctol 2020; 24(5):499. doi: 10.1007/s10151-020-02189-7.
- Grass F, Slieker J, Frauche P. Postoperative urinary retention in colorectal surgery within an enhanced recovery pathway. J Surgical Res 2017; 207:70-6. doi: 10.1016/j.jss. 2016.08.089.
- Werawatganon T, Charuluxanun S. Patient controlled intravenous opioid analgesia versus continuous epidural analgesia for pain after intra-abdominal surgery. Cochrane Database Systematic Rev 2005(1):Cd004088. doi: 10.1002/14651858.CD004088.pub2.
- Ushimaru Y, Kurokawa Y, Takahashi T. Is laparoscopic gastrectomy more advantageous for elderly patients than for young patients with resectable advanced gastric cancer? World J Surg 2020. doi: 10.1007/s00268- 020- 05486-2.
- Liu J, Meng Z, Lv R. Effect of intraoperative lung-protective mechanical ventilation on pulmonary oxygenation function and postoperative pulmonary complications after laparoscopic radical gastrectomy. Brazilian J Med Biological Res 2019; 52(6):e8523. doi: 10.1590/1414-431x20198523.
- Branch CMAS, Anesthesiology CMA. Enhanced recovery surgery chinese expert consensus and path management guidelines. Chinese J Anesthesiol 2018; 38(1):8-13. doi: 10.3760∕cma.j.issn.0254?1416.2018.01.003.
- Richardson K, Levett DZH, Jack S. Fit for surgery? Perspectives on preoperative exercise testing and training. British J Anaesthesia 2017; 119(suppl_1):i34-i43. doi: 10.1093/bja/aex393.
- Barberan-Garcia A, Ubré M, Roca J. Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery: A randomised blinded controlled trial. Ann Surg 2018; 267(1):50-6. doi: 10.1097/sla.0000 000000002293.
- Pisarska M, Torbicz G, Gajewska N. Compliance with the ERAS protocol and 3-year survival after laparoscopic surgery for non-metastatic colorectal cancer. World J Surg 2019; 43(10):2552-60. doi: 10.1007/s00268-019-05073-0.
- Rogers LJ, Bleetman D, Messenger DE. The impact of enhanced recovery after surgery (ERAS) protocol compliance on morbidity from resection for primary lung cancer. J Thoracic Cardiovascular Surg 2018; 155(4): 1843-52. doi: 10.1016/j.jtcvs.2017.10.151.
- rancis NK, Walker T, Carter F. Consensus on training and implementation of enhanced recovery after surgery: A delphi study. World J Surg 2018; 42(7):1919-28. doi: 10. 1007/s00268-017-4436-2.