Comparison of Uncultured Cell Spray and Split-Thickness Skin Grafting in Deep Partial-thickness Burns: A Novel Animal Model
By Safa Kursat Nural1, Omer Cennet1, Kemal Kosemehmetoglu2, Ali Konan1, Kaya Yorganci1Affiliations
doi: 10.29271/jcpsp.2023.06.673ABSTRACT
Objective: To compare uncultured cell spray and conventional surgery in deep second-degree burns in rats and create an experimental model for the use of this method.
Study Design: An experimental study.
Place and Duration of the Study: Hacettepe University Experimental Animals Application and Research Center, Ankara, Turkey, from October 2018 to December 2020.
Methodology: Twenty-four Wistar albino rats were divided into 4 groups. Two deep second-degree burns were created on the dorsal skin in different locations. On the 5th day of the burn wound, a split-thickness skin graft was applied to one of the burn wounds with half of the donor graft. Two-stage enzyme application was performed on the other half of the donor graft and keratinocytes were applied as a spray to the other tangential excision burn wound. Samples taken by excisional biopsy on certain days were examined macroscopically and histologically.
Results: In all the experimental groups according to sacrification days, macroscopic healing percentages, non-epithelised areas, inflammation and neovascularisation scoring were similar between graft side and spray side.
Conclusion: The effects of conventional split-thickness skin graft and uncultured cell spray on wound healing were comparable, suggesting that the uncultured cell spray method can be used as an alternative method to the classical burn treatment.
Key Words: Deep second-degree burn, Grafting, autologous cell, Non-cultured cell spray, Keratinocyte.
INTRODUCTION
Skin burns are treated according to their degree and extent. Since the epidermis and skin components are damaged in deep second-degree and higher-degree burns, healing occurs with scar tissue.1
The classical treatment of deep burns comprises the debridement of the burned wound and the closure of the defect with skin grafts taken from the intact part of the body.2 Complications such as infection, prolongation of hospitalisation, the emergence of aesthetic problems, and delayed healing can be seen in burn treatment.3 To minimise these complications, many auxiliary methods have been introduced including the transfer of autologous keratinocytes into the wound.4
Keratinocytes have a high proliferative capability, especially in the process of wound healing.5 Given that the keratinocyte-rich uncultured autologous cell spray method has many advantageous cosmetics and functional effects in the wound healing process, a method for implementation of uncultured autologous cells in patients has been reported recently.6,7 However, this method has been reported in the form of case reports, and clinical or experimental studies investigating the efficacy of this method are lacking.7
Due to the complex pathophysiology of burn injuries and their effects on various organ systems, in vitro experiments have been insufficient to explain the destructive effects of burns and improve the healing process, so animal experiments are needed.8 While many animals can be selected in experimental burn models, rats are one of the most preferred animals since they are small and easy to control. Rats are similar to humans in terms of many organ systems.9,10 However, there are some differences in terms of wound healing. Since wound contractions are rapid, healing time is reduced.11 This allows researchers to examine the wound healing mechanism quickly. This innovative approach to burn healing (uncultured cell spray) needs animal experiments before it can be accepted as a new treatment method for burns and can be compared with conventional methods. The aim of this study was to evaluate the method for implementation of uncultured autologous cells in the treatment of burns by creating an animal experimental model.
METHODOLOGY
This study was approved by Hacettepe University Animal Experiments Local Ethics Committee (Approval no: 2018/61). The experimental phase of the study was carried out at Hacettepe University Experimental Animals Application and Research Centre, from October 2018 to December 2020.
A total of 24 male Wistar albino rats weighing 250-350 grams were included in the study. Rats that died due to sepsis, burn shock, and dehydration were excluded from the study. Animals were fed with standard feed without water restriction in individual cages 10 days before being used in the experiment, and they were allowed to adapt to the environmental conditions. They were housed at a constant temperature, 12 hours of darkness, and 12 hours of light. Rats were randomly divided into 4 groups of 6 rats each group by using computer-generated chart (Microsoft Excel’s function RANDBETWEEN). Group 1 had standard wound care and follow-up for 3 days, postoperatively. Group 2 had standard wound care and follow-up for 7 days, postoperatively. Group 3 had standard wound care and follow-up for 14 days, postoperatively, and Group 4 had standard wound care and follow-up for 21 days, postoperatively.
Subjects were anaesthetised by intraperitoneal administration of a mixture of 90 mg/kg ketamine hydrochloride (Ketalar®, Eczacıbaşı, İstanbul, Turkey) and 10 mg/kg Xylazine HCl (Alfazyne® 2%, 20 mg/ml, 30 ml, Alfasan Int B V. Netherlands). After shaving the dorsal skin of the anaesthetised rats, an aluminium plate weighing 51 grams with a surface area of 2 cm² was dropped into boiling water heated to 100°C under thermometer control, by the previously described method, to create a second-degree deep burn. As a result of contact with the weight of the plate for 15 seconds on the dorsal part of the rats, two deep second-degree burns of approximately 2 cm² were created.12,13 Immediately afterwards, metamizole sodium (Onpyron®, Onfarma, Samsun) 40 mg/kg was administered subcutaneously to the dorsal neck for analgesia, followed by oral administration of 200 mg/Kg of metamizole sodium mixed with drinking water. In addition, 5 ml of saline was injected subcutaneously into the dorsal neck for hydration.
Subjects were reprocessed 5 days later for tangential excision, skin- and spray-grafting. After anaesthesia, the donor area was disinfected with chlorhexidine (Dermanios Scrub®, Anios). A partial-thickness skin graft of 4×1 cm was taken from the right inguinal region with the help of a scalpel. The skin graft taken was divided into two in the middle and two 2×1 cm skin grafts were obtained. Tangential excision was made with a hand dermatome (Wecprep™ Blades, Pilling Weck Surgical, PA, USA) on both of the formed burned tissue.
A split-thickness skin graft was applied to the upper part of the burned areas. The graft was fixed with a 4.0 monofilament absorbable suture (Maxon™ Covidien).
The remaining 2x1 cm part of the skin graft was taken into a sterile container and cut into small pieces. The specimen was incubated for 40 minutes in a container containing Dispase II (Dispase II Neutral Protease, NC, USA) solution until the dermis-epidermis connection was separated. Then, it was soaked into Ringer's lactate solution to remove the enzyme. The epidermis and dermis were mechanically separated with the help of a scalpel and forceps. The epidermis was kept in a tube containing Trypsin/EDTA (Trypsin EDTA Solution C 0.05/0.02%, Biological Industries, USA) solution for 15 minutes, shaking occasionally. Then, the specimen was washed with Ringer's lactate solution to remove the enzyme. The cell suspension was passed through a 70-micrometer cell strainer (70µM Cell Strainer, Orange Scientific, Belgium). This suspension containing regenerative basal keratinocyte is added to Ringer's lactate and centrifuged at 200 g for 5 minutes. The ringer lactate remained in the upper part of the tube after centrifugation was discarded. A fresh Ringer's lactate solution was added to the suspension containing regenerative basal keratinocytes, which remained precipitated in the tube. The obtained keratinocyte-rich solution was applied to the burn wound with the help of a syringe. The subjects were taken into individual cages after the procedure. After then, routine wound care was continued in the form of dressing in petrolatum-impregnated sterile gauze (Xeroform®, Covidien, USA).
After the groups were sacrificed with the high-dose anaesthetic agent on the 3rd, 7th, 14th, and 21st postoperative days, biopsies were taken from intact skin and from two sites on which skin grafts and uncultured cell spray were applied.
Pathological findings were assessed by one of the authors blinded to group allocations. After sacrification, all subjects were macroscopically evaluated by direct observation method and their photographs from the same angle and distance were taken. Wound healing in all groups was evaluated by the measurement of the necrotic area. It was scored as 1 if the healing is detected in more than half of burned area; 0 if necrosis was seen. The percentage area of macroscopic healing was also recorded as described.14
Samples were taken parallel to the long axis of the burned areas. They were fixed with 10% buffered formaldehyde and 5-mm-thick sections were stained with routine hematoxylin-eosin. Tissues were evaluated semi-quantitatively in terms of wound healing findings (non-epithelialised area, neovascularisation, inflammation). These parameters were scored histologically by giving a score between 0-3 in terms of neovascularisation and inflammation (Table I).15
Data were analysed using SPSS version 23.0 (IBM Corporation, Armonk, NY, USA). During the evaluation of the data collected in the study, normality tests were performed with the Shapiro Wilk's test. When the Q-Q plot graphics were examined, non-parametric methods were preferred. Wilcoxon test was used for the macroscopic analysis. Correlation tests were performed with the Spearman test. A p-value of less than 0.05 was considered to show a statistically significant result.
Table I: Histological scoring system.
|
|
0 |
1 |
2 |
3 |
|
Acute Inflammation |
None |
Scant |
Moderate |
Abundant |
|
Neovascularization |
None |
<5 /HPF |
6-10 /HPF |
>10 /HPF |
Table II: Median histological scores of graft and spray groups.
|
Acute Inflammation |
Neovascularisation |
|||||
|
|
Graft |
Spray |
p* |
Graft |
Spray |
p* |
|
Day 3 |
2 |
1.5 |
0.06 |
1 |
2.5 |
0.71 |
|
Day 7 |
3 |
2 |
0.57 |
1.5 |
2.5 |
0.48 |
|
Day 14 |
2 |
1 |
0.71 |
1 |
3 |
0.45 |
|
Day 21 |
2.5 |
1 |
0.56 |
2 |
1.5 |
0.16 |
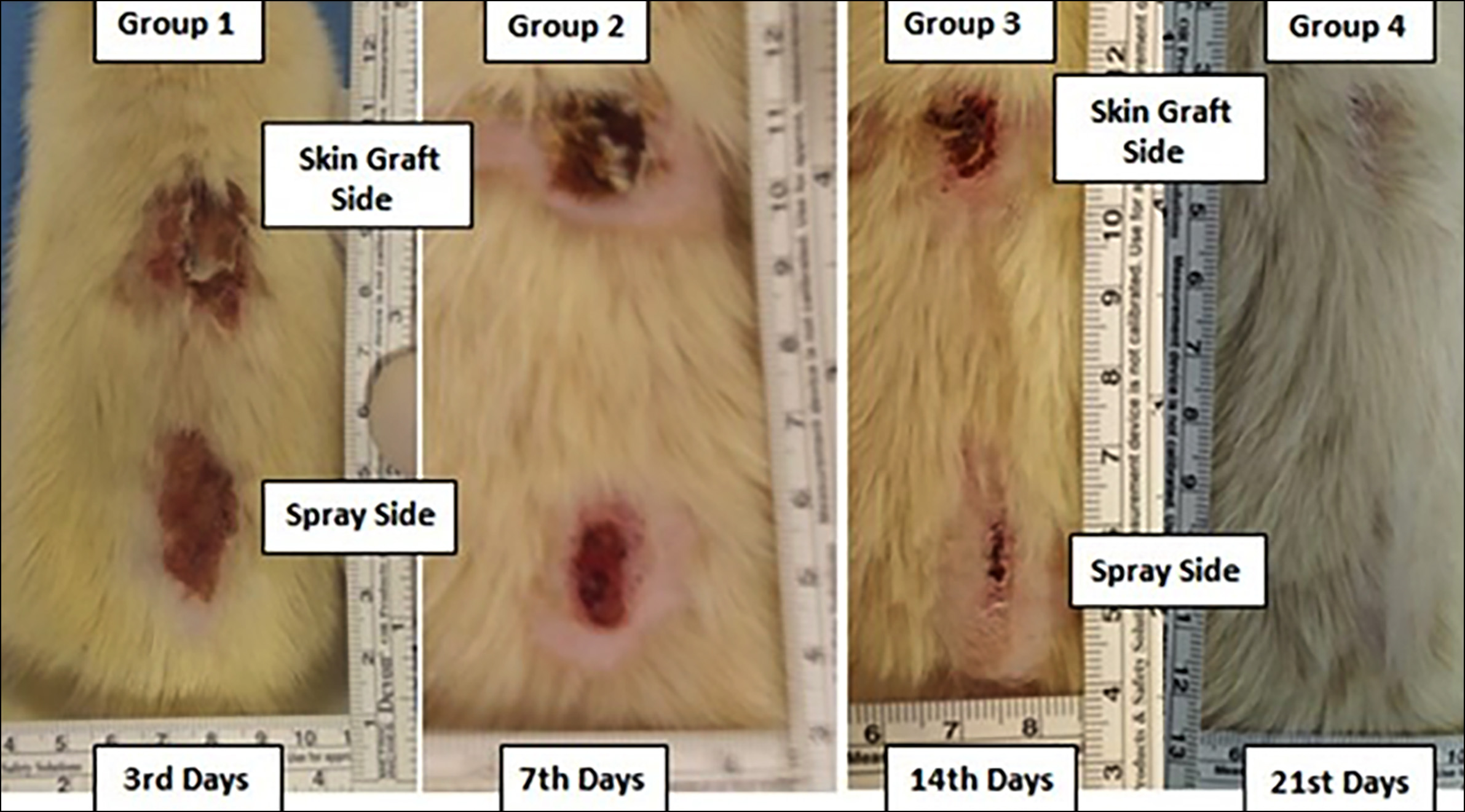 Figure 1: Macroscopic appearance of spray and skin graft in burn wounds on the 3rd, 7th, 14th, and 21st days after the procedure.
Figure 1: Macroscopic appearance of spray and skin graft in burn wounds on the 3rd, 7th, 14th, and 21st days after the procedure.
RESULTS
Before sacrification, 4 rats - two rats from group 3 and another two rats from group 4- died of procedural complications (shock and sepsis). Therefore, the remaining 20 subjects were evaluated for macroscopic analysis, amount of non-epithelialised area, inflammation, and neovascularisation.
When skin-grafted and spray-applied sides were compared according to days, there was no statistically significant difference between areas of healing (Figure 1). The difference was the greatest on the 14th day [spray side median (IQR) 90% (85-95) vs. skin graft side 75%(60-80)]; however, it was equalised at the end of the 21st day [spray side median (IQR) 100% (90-100) vs. skin graft side 92.5% (90-97.5)]. Overall, the median (IQR) percentages of macroscopic healing of the subjects were 80% (65-90) vs. 70% (50-80) on spray and skin graft applied sides, respectively (p=0.011).
Regarding the sacrification day, the non-epithelialised area of the skin graft and spray-applied sides were statistically indifferent; however, the difference was the greatest on the 3rd day [(13.5 mm (2-20) vs. 20mm(20-21), respectively]. Overall, the median (IQR) values of non-epithelialised areas were 11.5 (2.5-18.5) mm in skin-grafted side compared to 8 mm (1-15.5) in spray-applied side (p=0.192).
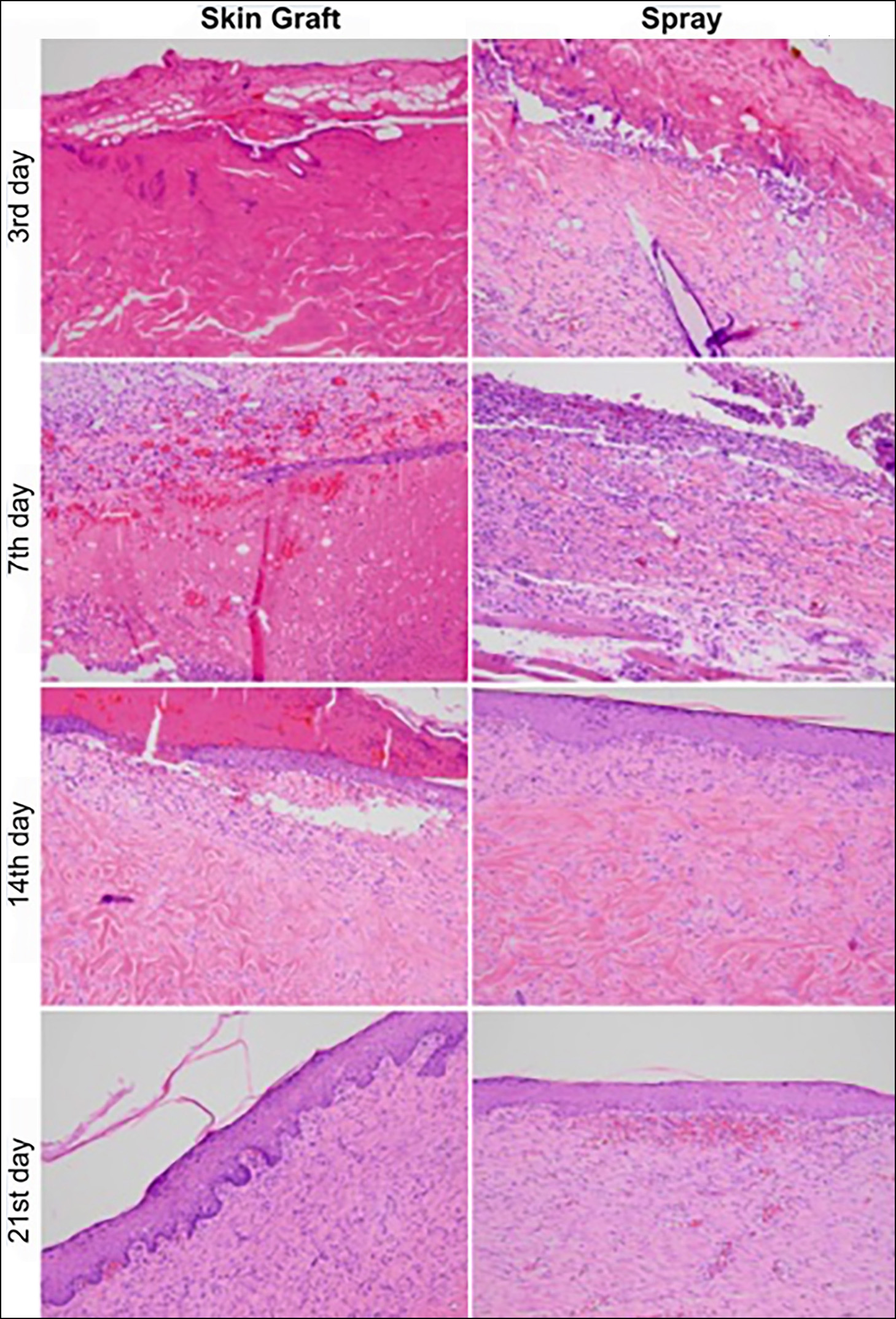 Figure 2: Histological appearances of the skin graft and spray side of the groups according to sacrification day. Morphological landmarks of healing process was comparable in both groups.
Figure 2: Histological appearances of the skin graft and spray side of the groups according to sacrification day. Morphological landmarks of healing process was comparable in both groups.
There was no significant difference between neovascularization or inflammation scores of spray and skin graft sides (Table II and Figure 2). Subjects were also compared with the percentage of macroscopic healing and non-epithelialised area. There was a moderately strong, negative correlation between the percentage of macroscopic healing and the size of the non-epithelialised area (r= -0.52, p=0.016, Spearman's test).
DISCUSSION
Burn cases can be complicated depending on the surface area size and depth and may result in death. While first and superficial second-degree burn wounds can only heal with wound care; deep second and third-degree burns require surgical treatment. Closure of burn wounds by the use of autograft is the standard treatment method for these patients. Nevertheless, the search for ideal treatment continues due to problems such as requiring long-term wound care, infection, donor site care, and complications.16 Therefore, the use of uncultured cell spray as an alternative treatment has become the main topic of interest in recent years.6,7 However, studies investigating the effectiveness of this method against classical burn wound treatment are not sufficient.17 In order to contribute to the studies on this subject, the authors planned to investigate whether uncultured cell spray with an animal experimental model could be a method that can replace the classical treatment. The aim of this study was to examine an alternative and innovative burn treatment method with animal experimentation in terms of complications and the healing process.
The literature was used to obtain the appropriate burn model. Pereira et al. reported some criteria to provide standardisation in the creation of deep second-degree burns in rats. To create the burn model, they prepared an aluminium plate weighing 51 grams and kept this plate in boiling water heated to 100 °C. In their histological and macroscopic studies, they observed that deep second-degree burns occurred in this way.18 While creating the wounds, the aluminium plates were left with their weight without the application of any additional pressure.13 When the subjects were re-examined on the 5th day of the procedure, a deep second-degree burn was observed. When the literature was examined again, it was seen that the macroscopic and histopathological changes in the burn wound were more evident on the 3rd, 7th, 14th and 21st days, respectively, in animal experiments, and the studies were more appropriate. For this reason, these days were selected in this study.18
Skin regeneration is a dynamic process in which progenetic cells reorganise tissue structure and function, led by different cell types and cell signalling mechanisms. It is thought that using the patient's autologous cells accelerates healing and increases the quality of recovery.7,19 Esteban et al. listed the advantageous aspects of uncultured cell spray as requiring minimal cell manipulation, reducing progenitor and epidermal stem cell loss, and using the patient's tissue as a bioreactor.7 Gerlach et al. reported that regenerative basal keratinocytes in the epidermis cannot be isolated by conventional methods. He argued that these cells could be revealed.19 In this study, the authors also applied this method to rats and created an experimental rat model with uncultured cell spray.
This innovative approach originally emerged as the application of cultured cells. Although cultured epidermal autografts have been used for some time; the process may take longer and the results may not be satisfactory. Besides, the use of differentiated keratinocytes often results in cosmetically inadequate regeneration.20 The uncultured cell spray method put an end to the problem of the long processing time spent during culture. Moreover, it reduced the amount of in vitro cell manipulation and, more importantly, prevented early cell differentiation with loss of progenitor cells and epidermal stem cells.
Today, classical surgical treatment is applied in the form of early tangential excision and split-thickness skin graft. However, this classical surgical treatment modality causes many problems. The main problems can be listed as infection, long hospital stays due to the need for long-term wound care, donor site problem in patients with extensive burns and functional and aesthetic losses. Esteban et al. reported that the uncultured cell spray method was faster re-epithelialisation than conventional autografting.7 Gravante et al. argued that epithelialisation develops faster in the uncultured cell spray method, and it is easier to use in burns in specific areas.21 In addition, studies showed that the use of uncultured cell spray and split-thickness skin graft together is more effective in wound healing in adult patients.22 However, the experimental studies and infrastructure of this method in the literature are insufficient. Filling this gap, this study provided a model for the treatment of burns with the spray method and suggested a resource for further studies in this field.
In this study, wound healing in the spray and autologous skin graft were comparable both macroscopically and microscopically. Interestingly, the percentage of macroscopic healing and histological scores on the skin graft side was inferior to the spray group in the first days. However, this gap closed in the following days. This might be due to the higher complication rate (graft infection, necrosis, etc.) in skin grafting. Another explanation could be the better contact of the solution applied in the form of a spray with the underlying tissue. Spray application is proved to achieve successful results similar to skin grafting with lower complications compared to skin graft application even with an advantage in the early period.
Neovascularisation is a very important process in wound healing. Deep burns require neovascularisation for healing to occur. In a study conducted on deep burns, it was stated that the fluid in burn blisters contributes to neovascularisation and its evaluation is an indicator of burn healing.23 The neovascularisation and inflammation scores between the two groups in this model had no statistically significant difference suggesting that uncultured autologous cell spray can be as effective as a standard treatment and may be used as an alternative effective treatment method.
In this study, a useful animal model of uncultured cell spray was proposed, and comparable results in the healing process of second-degree deep burns in conventional autograft application were obtained with uncultured cell spray compared to standard treatment. The results of treatment methods based on the use of progenitor stem cells are quite exciting. Gaps in the literature indicate that these new treatment approach modalities need to be developed. This study, which has similar aspects with some methods used in clinical applications (eg. ReCell®), is also very important because of the creation of an experimental animal model.24 On the other hand, there are some limitations of this study. The nutrition of the grafts is only provided by diffusion from the underlying tissues during the first four days.25 Therefore, contact of the graft with a well-blooded tissue underneath is essential for the survival of the graft. In this model, a burn area was created in the midline with the vertebrae. This area may have made it difficult for the conventional skin graft to adhere to some areas of the wound. Since non-cultured autologous cells in these areas were applied to the wound in liquid-spray form, contact with the tissue may have been better. The comparison of uncultured autologous cell spray and split-thickness skin graft in different parts of the animal body may be an interesting subject of a new study.
CONCLUSION
Uncultured cell spray was observed to be similarly successful with the application of split-thickness skin grafts in the healing process of deep second-degree burns. The results were close to each other in the parameters examined. This innovative research tried to be examined more objectively with an animal experimental model. However, more studies are needed before it can be used as a completely alternative treatment method.
ETHICAL APPROVAL:
This study was approved by Hacettepe University Animal Experiments Local Ethics Committee (Approval No. 2018/61).
PATIENTS' CONSENT:
Patients' consent was not obtained because it was an experimental animal study.
COMPETING INTEREST:
The authors declare that they have no competing interest with respect to authorship and publication of this article.
AUTHORS’ CONTRIBUTION:
SKN, AK: Conceptualisation.
SKN, OC: Data curation.
SKN, OC, KK, AK, KY: Methodology.
OC, SKN, KK: Writing-original draft.
AK, KY: Writing-review and editing.
All authors have read and approved the final manuscript.
REFERENCES
- Daigeler A, Kapalschinski N, Lehnhardt M. Therapy of burns. Chirurg 2015; 86(4):389-401 doi: 10.1007/s00 104-014-2919-3.
- Sayek I. Temel Cerrahi: Gunes Tip Kitabevi; 2013.
- Zerbaliyev E, Gultekin Y, Erkent M, Konan A, Yorganci K. Improvement in treatment efficacy in patients admitted to the hacettepe university burn unit-33 years of experience. J Crit Inten Care 2016; 7:77.
- Acikel C, Eren F, Çeliköz B. Bir Yanık ünitesinde yatarak tedavi edilen akut yanuklı hastaların maliyeti. Türk Plastik, Rekonstrüktif ve Estetik Cerrahi Dergisi (Turk J Plast Surg) 2002; 10:186-9.
- Kose O. Epidermal Kok Hucreler. Turk J Dermato/Turk Dermatoloji Dergisis 2015; 9.
- Gerlach JC, Johnen C, McCoy E, Brautigam K, Plettig J, Corcos A. autologous skin cell spray-transplantation for a deep dermal burn patient in an ambulant treatment room setting. Burns 2011; 37:e19-23 doi: 10.1016/j.burns. 2011.01.022.
- Esteban-Vives R, Choi MS, Young MT, Over P, Ziembicki J, Corcos A, et al. Second-degree burns with six etiologies treated with autologous noncultured cell-spray grafting. Burns 2016; 42(7):e99-e106 doi: 10.1016/j.burns.2016 .02.020.
- Zhai Q, Zhou F, Ibrahim MM, Zhao J, Liu X, Wu J, et al. An immune-competent rat split thickness skin graft model: useful tools to develop new therapies to improve skin graft survival. Am J Transl Res 2018; 10(6):1600-10.
- Rosenthal N, Brown S. The mouse ascending: perspectives for human-disease models. Nat Cell Biol 2007; 9(9):993-9 doi: 10.1038/ncb437.
- Mitsunaga Junior JK, Gragnani A, Ramos ML, Ferreira LM. Rat an experimental model for burns: A systematic review. Acta Cir Bras 2012; 27(6):417-23 doi: 10.1590/ s0102-86502012000600010.
- Dorsett-Martin WA. Rat models of skin wound healing: A review. Wound Repair Regen 2004; 12(6):591-9 doi: 10. 1111/j.1067-1927.2004.12601.x.
- Abdullahi A, Amini-Nik S, Jeschke MG. Animal models in burn research. Cell Mol Life Sci 2014; 71(17):3241-55 doi: 10. 1007/s00018-014-1612-5.
- Venter NG, Monte-Alto-Costa A, Marques RG. A new model for the standardization of experimental burn wounds. Burns 2015; 41(3):542-7 doi: 10.1016/j.burns. 2014.08.002.
- Chatraie M, Torkaman G, Khani M, Salehi H, Shokri B. In vivo study of non-invasive effects of non-thermal plasma in pressure ulcer treatment. Sci Rep 2018; 8(1):1-11. doi: 10.1038/s41598-018-24049-z.
- Abramov Y, Golden B, Sullivan M, Botros SM, Miller JJ, Alshahrour A, et al. Histologic characterization of vaginal vs. abdominal surgical wound healing in a rabbit model. Wound Repair Regen 2007; 15:80-6 doi: 10.1111/j.1524- 475X.2006.00188.x.
- O'Brien SP, Billmire DA. Prevention and management of outpatient pediatric burns. J Craniofac Surg 2008; 19: 1034-9 doi: 10.1097/SCS.0b013e318177217c.
- Rowan MP, Cancio LC, Elster EA, Burmeister DM, Rose LF, Natesan S, et al. Burn wound healing and treatment: Review and advancements. Crit Care 2015; 19:243 doi: 10.1186/ s13054-015-0961-2.
- Tavares Pereira Ddos S, Lima-Ribeiro MH, de Pontes-Filho NT, Carneiro-Leao AM, Correia MT. Development of animal model for studying deep second-degree thermal burns. J Biomed Biotechnol 2012; 2012:460841 doi: 10.1155/ 2012/460841.
- Gerlach JC, Johnen C, Ottomann C, Brautigam K, Plettig J, Belfekroun C, et al. Method for autologous single skin cell isolation for regenerative cell spray transplantation with non-cultured cells. Int J Artif Organs 2011; 34(3):271-9 doi: 10.5301/ijao.2011.6508.
- Chester DL, Balderson DS, Papini RP. A review of keratinocyte delivery to the wound bed. J Burn Care Rehabil 2004; 25(3):266-75 doi: 10.1097/01.bcr.0000 124749.85552.cd.
- Gravante G, Di Fede MC, Araco A, Grimaldi M, De Angelis B, Arpino A, et al. A randomised trial comparing ReCell system of epidermal cells delivery versus classic skin grafts for the treatment of deep partial thickness burns. Burns 2007; 33(8):966-72 doi: 10.1016/j.burns.2007. 04.011.
- Nanassy AD, Glat PM, Burkey BA, Misra AC, Meyer LK, Gates L, et al. The use of an autologous cell harvesting and processing device to decrease surgical procedures and expedite healing in two pediatric burn patients. Wounds 2019; 31(12):316-21.
- Pan SC, Wu LW, Chen CL, Shieh SJ, Chiu HY. Deep partial thickness burn blister fluid promotes neovascularisation in the early stage of burn wound healing. Wound Repair Regen 2010; 18(3):311-8 doi: 10.1111/j.1524-475X.2010. 00586.x.
- Holmes Iv JH, Molnar JA, Carter JE, Hwang J, Cairns BA, King BT, et al. A comparative study of the ReCell® device and autologous split-thickness meshed skin graft in the treatment of acute burn injuries. J Burn Care Res 2018; 39(5):694-702. doi: 10.1093/jbcr/iry029.
- Braza ME, Fahrenkopf MP. Split-thickness skin grafts. Stat pearls. Treasure Island (FL)2021.