Combination Treatment with Iodine 125 Seeds Implant and Systemic Therapy vs. Systemic Therapy Alone for Non-small Cell Lung Cancer: A Systematic Review and Meta-analysis
By Huzi Li1, Wentao Li1, Libo Zhang2, Wenyan Fang1, Hong Zhang1Affiliations
doi: 10.29271/jcpsp.2023.01.88ABSTRACT
Combination treatment with iodine 125 seeds implant and systemic therapy in patients with non-small-cell lung cancer (NSCLC) is a promising treatment practice. The present study aimed to assess the relative efficacy and toxicity of combination treatment versus systemic therapy alone in patients with NSCLC. Databases including PubMed, EBSCO, Web of Science, EMBASE, Cochrane Library, CNKI, and WanFang were searched for relevant randomised controlled trials (RCTs). Risk ratios (RR) were obtained for evaluating indicators in the present meta-analysis including complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD), overall response rate (ORR), disease control rate (DCR), one-year and two-year overall survival (OS) rate and complications.
A total of 17 eligible RCTs incorporating 1315 patients who underwent combination treatment or systemic therapy alone were ultimately included in this meta-analysis based on our selection criteria. The results showed that CR (RR = 1.89, 95% confidence interval [CI]: 1.53 - 2.33, p <0.001), PR (RR = 1.28, 95%CI: 1.12 - 1.46, p = 0.0002), ORR (RR = 1.46, 95%CI: 1.34 - 1.58, p <0.001), DCR (RR = 1.11, 95%CI: 1.04 - 1.18, p = 0.001), two-year OS (RR = 1.52, 95% CI: 1.30 - 1.77, p <0.001) were higher and SD (RR = 0.53, 95%CI: 0.42 - 0.66, p <0.001) and PD (RR = 0.39, 95%CI: 0.29 - 0.55, p <0.001) were lower in the combination treatment group than in control group. Meanwhile, there was no significant difference in one-year OS (RR = 1.13, 95% CI: 0.98-1.31, p = 0.10). In terms of adverse events, the combination therapy significantly increased the incidence of pneumothorax (RR = 4.91, 95% CI: 2.63 – 9.17, p <0.001); however, no significant differences were found in the incidence of myelosuppression and gastrointestinal symptoms.
Combination treatment with iodine 125 seeds implant and systemic therapy can significantly improve clinical response and prolong two-year OS in NSCLC patients without increasing the incidence of myelosuppression and gastrointestinal symptoms, except pneumothorax.
Key Words: Brachytherapy, Radioactive seeds, NSCLC, Systemic therapy.
INTRODUCTION
With the development of computerised three-dimensional planning system, interstitial implantation of radioactive seeds has been the focus for treating malignant tumors in recent years.1 It, served as stereotactic ablation brachytherapy (SABT),2 has been a novel therapy modality for inoperable cancers,3 with a satisfactory local control rate.4-7
Implantation of radioactive seeds, a common treatment practice guided by computed tomography (CT), ultrasound or bronchscopy, has technical superiority of achieving a high radiation dose in a single fraction to the target8,9 to continuously destroy tumour cells, and better organ sparing to protect surrounding normal tissues from radiation damage, as compared to traditional external beam radiation therapy4. Additionally, it has been shown that apoptosis-related genes could be up-regulated by radioactive seeds to induce apoptosis of tumour cells in vitro studies.10,11
Interstitial brachytherapy is often used in combination with or following systemic therapy in patients with non-small cell lung cancer (NSCLC).12-14 Although systemic therapies including traditional chemotherapy, tyrosine kinase inhibitors (TKIs), immune checkpoint inhibitors (ICI) and so on, remain the first-line and indispensable treatment for inoperable NSCLC patients, previous findings suggested that internal radiotherapy with radioactive iodine-125 seeds may be an alternative modality for all kinds of NSCLC patients.3,12,15-17 It has been proven to show good effects on improving local control rate and decreasing local recurrence and could have a better prognosis for locally advanced or metastatic NSCLC patients. It was postulated that combination treatment with iodine 125 seeds implant and systemic therapy could be superior to systemic therapy alone. Three previous meta analyses have been published to assess the efficacy and safety of the combination treatment with seeds implant and systemic chemotherapy.16,18,19 All these pooled analyses have shown the improvement of short-term efficacy in the group of combination treatment over chemotherapy alone; however, the pooled outcomes were not consistent among the studies, and two-year OS was not shown to be significantly different between two groups in Qiu et al.’s report,19 while statistically significant difference was found in the other two meta-analyses. Trials included in the meta-analyses performed by Wu et al.,18and Zhang.et al.,16 were mixed with randomised controlled trials (RCTs) and non-RCTs. However, comparability could not be ensured between different groups before treatment and it is difficult to avoid selective bias due to non-randomisation. In Qiu et al.’s trial, five RCTs were included, however, among the included five trials, there was a retrospective study6 and groups were assigned according to voluntary willingness to accept treatment for enrolled patients in another trial,20 and in the third study comparability of baseline characteristics was not reported,21 leading to potential bias. Additionally, several Chinese records in two studies were pooled-analysed without Chinese literature as well as the same search strategy in English,18,19 which may also lead to publication bias. So, its efficacy and safety remain unclear and more convincing evidence to evaluate the efficacy and safety of combination treatment vs. systemic therapy alone is still clinically required through pooled analyses to provide the latest and most comprehensive evidence to guide clinical trials for advanced NSCLC in future.
The application and research about brachytherapy with iodine-125 radioactive seeds in NSCLC patients is increasing, and several RCTs havebeen carried out in recent years. The aim of was to analyse the clinical efficacy of the combination therapy with iodine 125 implant and chemotherapy for the treatment of lung cancer by using meta-analysis method.
METHODOLOGY
Literature from PubMed, EBSCO, Web of Science, EMBASE, Cochrane Library, China Knowledge Resource Integrated Database (CNKI), Wan Fang Database was searched and included in this meta-analysis from January 2005 to November 2020, according to guidelines from the Cochrane Collaboration. The publication languages were restricted to Chinese and English. The main medical terms used for literature search were “Lung cancer or lung carcinoma or NSCLC” and “Iodine-125 or I125 or 125I or brachytherapy”. The references of the included studies were also screened manually.
In this analysis studies eligible for inclusion were (i) Studies must have been designed as randomised controlled trials (RCTs); (ii) Patients must have been clinically or pathologically diagnosed as NSCLC, including primary tumour or progression and recurrence after previous treatment; (iii) Treatment of the control group was systemic therapy alone including chemotherapy, targeted therapy with tyrosine kinase inhibitors, immune checkpoint inhibitors or traditional medicine; (iv) Treatment of the experimental group was implantation with radioactive iodine-125 seeds guided by computerized tomography(CT), ultrasound or endoscope, combined with the same systemic therapeutic regimen as the control group; (v) There were necessary detailed basic characteristics of the enrolled patients with no statistical difference between the experimental group and the control group; (vi) Outcome was reported including tumour response evaluation according to World Health Organisation criteria or Response Evaluation Criteria in Solid Tumours, overall survive rate or adverse events.
The major exclusion criteria were: (i) retrospective control studies; (ii) single-arm studies; (iii) patients with metastatic lung cancer, multiple primary tumours, small cell lung cancer, or other cancers; (iv) no clinical studies or no human trials including cytological experiments, animal studies or other pre-clinical trails; (v) patients who have received synchronous other local treatment including resection, external beam irradiation, cryoablation, radiofrequency ablation, microwave ablation, regional perfusion chemotherapy though tumour blood vessels, and so on; (vi) reviews, meta-analysis, guidelines, expert consensus, case reports or repeated reports; (vii) lack of detailed data about general characteristics or lack of primary results from the published studies and connection with authors; and (viii) other irrelevant studies.
Two investigators (HZ Li, LB Zhang) independently selected potentially relevant articles on the basis of the inclusion and exclusion criteria, and a third reviewer (L Zhang) was involved to resolve the disagreements between the two reviewers. According to the PRISMA guidelines, the selection process is presented in the flow chart (Figure 1). Two investigators (HZ Li, WT Li) conducted data extraction from the included studies, using a standardised data collection form. The data abstracted included publication details, demographic and clinical information; intervention factors and prescription dose; and outcome measures including complete response (CR), partial response (PR), stable disease or no change (SD), progressive disease (PD), one-year OS rate, two-year OS rate and several most frequent adverse events. And overall response rate (ORR) was defined as sum of CR and PR; disease control rate (DCR) was defined as as CR+PR+SD.
The general methodological quality of each enrolled study was evaluated according to the RCT quality evaluation standards of the Cochrane handbook. Two reviewers (LB Zhang, H Zhang) independently completed and assessed the quality of the included studies based on:(i) random sequence generation (selection bias); (ii) allocation concealment (selection bias);(iii) blinding of participants and personnel (performance bias); (iv) blinding of outcome assessment (detection bias); (v) incomplete outcome data (attrition bias); (vi) selective reporting (reporting bias);and (vii) other biases. All disagreements were resolved by discussions among the investigators and a consensus was reached.
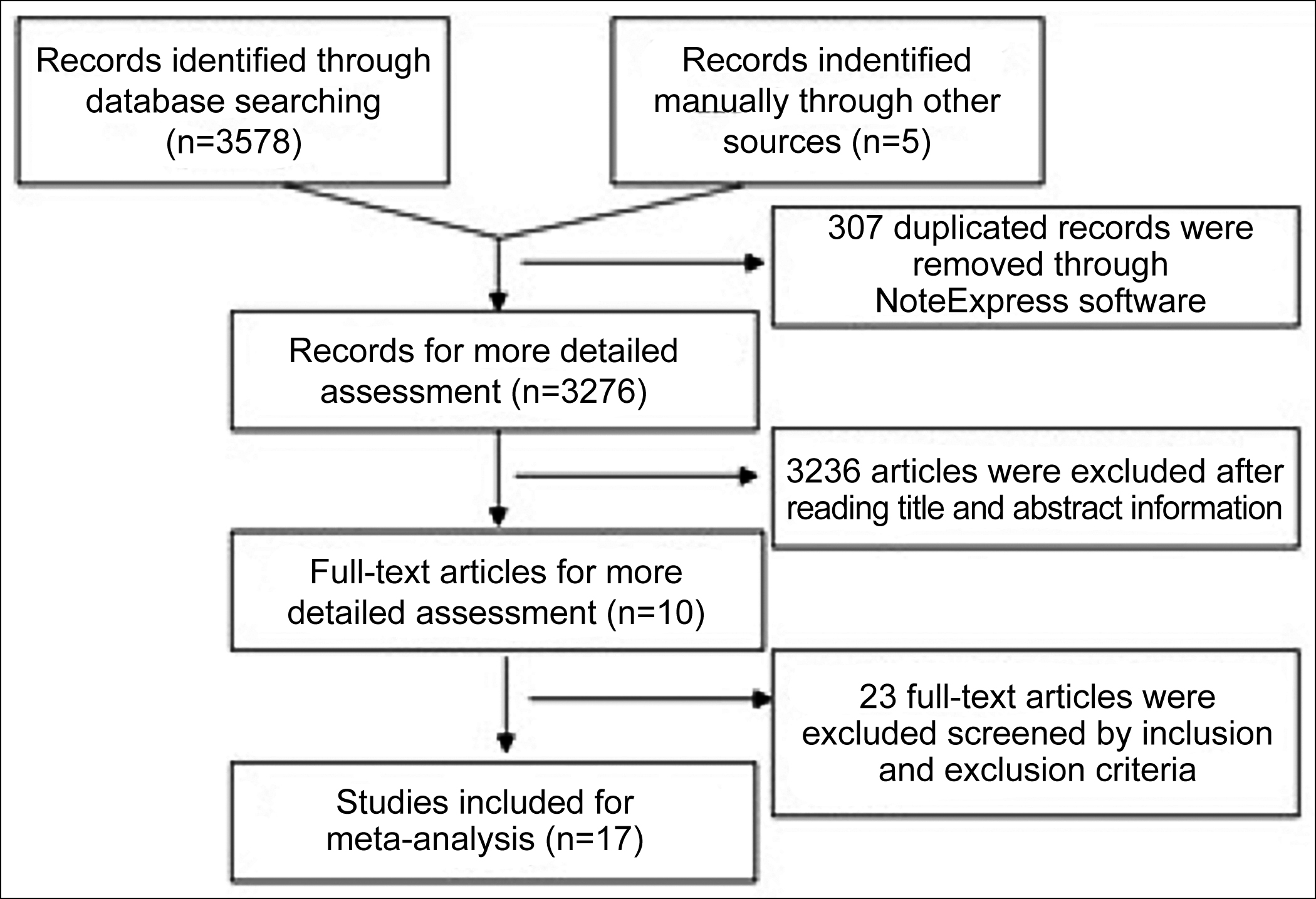 Figure 1: Flow diagram of the study selection process.
Figure 1: Flow diagram of the study selection process.
The meta-analysis was performed with Review Manager software (version 5.3) and R software (version 4.0.2). We calculated relative risk ratio (RR) with 95% confidence interval (95%CI) as indicators of efficacy and safety. Heterogeneity among studies was evaluated by I2 statistics and p-values. We chose a fixed-effects method with a predefined significance threshold of I2 <50% and p >0.1, otherwise a random-effect method was used. If heterogeneity existed, its sources were analyzed and subcategory analysis was adopted for factors that could have contributed to the heterogeneity. Publication bias in this study was tested by funnel plots with Egger’s test at a level of 0.1. For all tests p value 0.05 was considered statistically significant.
RESULTS
According to the initial search strategy, a total of 3583 potentially relevant studies were identified during the initial literature search, and the process of literature search is summarized in Figure 1. After abandoning duplicate and non-available articles filtered by inclusion and exclusion criteria, remaining 17 RCTs involving 1315 patients were successfully selected for the present systematic review and meta-analysis, among these paitents 660 in the experimental group while 655 belonged to control group.22-38
The main characteristics of the included RCTs were summarized in Table I. The general data extracted from all included trials indicated statistically comparable between the two groups. Of all the included RCTs, random digital table was performed in 10 trials23-25,30,32,34-38 and random draw in one trial,28 as randomisation method.The quality evaluations and bias risk of the included studies is presented in Figure 2.
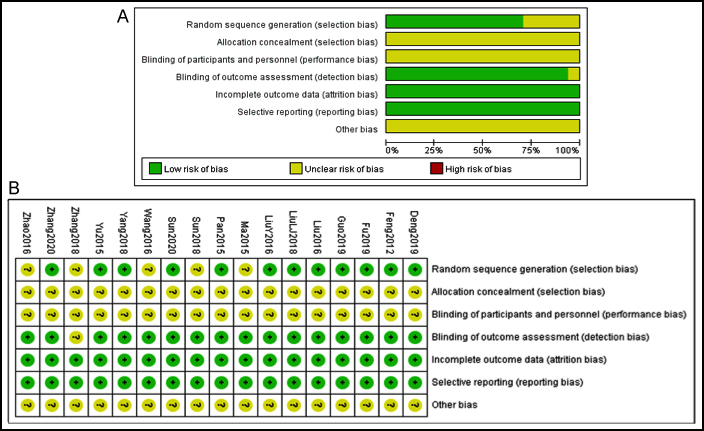 Figure 2: Risk of bias summary diagram and risk of bias percentile chart.
Figure 2: Risk of bias summary diagram and risk of bias percentile chart.
To systematically review the overall short-term efficacy, evaluating indicators including CR, PR, SD, PD, ORR and DCR were measured to assess responses among 16 RCTs22-25,27-38 (Figure 3).
There was no significant heterogeneity among 16 trials for CR (I2 = 16%, p = 0.27), PR (I2 = 0%, p = 0.70), SD (I2 = 0%, p = 0.67), PD (I2 = 0%, p = 0.76), and ORR (I2 = 7%, p = 0.37) , respectively, then the fixed effects models were used to calculate RR and 95%CI. Outcome indicated that CR (RR = 1.90, 95%CI: 1.53 - 2.36, p <0.001), PR (RR = 1.28, 95%CI: 1.12 - 1.46, p = 0.0002), and ORR (RR = 1.46, 95%CI: 1.34 - 1.58, p <0.001) in the experimental group were significantly higher than control group, while SD (RR = 0.53, 95%CI: 0.42 - 0.66, p <0.001) and PD (RR = 0.39, 95%CI: 0.29 - 0.55, p <0.001)were higher in the control group on the contrary.
By contrast, heterogeneity among included studies was found in the analysis of DCR (I2 = 69%, p <0.001), and pooled analysis revealed that the DCR was statistically different from a random effects model (RR = 1.11, 95%CI: 1.04 - 1.18, p = 0.001) between the two groups.
Overall survival:
The meta-analysis results of long-term therapeutic efficacy are shown in Figure 4. For one-year OS rate, the random-effects model was performed to calculate RR and 95% CI, with significant heterogeneity (I2 = 76%, p = 0.0004) among the included RCTs.22,23,26,33-36 Results suggested that the one-year OS in the combination group was not significantly different between two groups (RR = 1.13, 95% CI: 0.98-1.31, p = 0.10). For two-year OS, no significant heterogeneity was detected among the included 7 studies22,23,26,27,33,35,36 (I2 = 0%, p = 0.65), hence, RR and 95% CI were calculated by the fixed-effects model. Our pooled-analysis suggested that it was obviously higher in experimental group than in the control group (RR = 1.52, 95% CI: 1.30 – 1.77, p <0.001).
Toxicity and side effects:
For the safety meta-analysis, we selected the most commonly reported adverse events, including pneumothorax (reported in 12 RCTs23-28,30,32-35,38), myelosuppression (reported in 7 RCTs25,30,32-35,37) and gastrointestinal symptoms (reported in 10 RCTs25,27,28,31-37).
Table I: General characteristics of all included studies.|
Trials/Groups |
N |
Gender |
Age (years) |
Histology |
Stage |
Treatment |
Comparability |
||||
|
Male |
Female |
mean (range) |
SCC |
ADC |
Other |
||||||
|
Ma201522 |
Con |
42 |
29 |
13 |
62.8 (51-75) |
9 |
25 |
8 |
III/IV |
Chemotherapy |
Y |
|
Exp |
41 |
27 |
14 |
63.9 (53-76) |
8 |
26 |
7 |
Combination |
|||
|
Pan201523 |
Con |
40 |
22 |
18 |
62.1 (45-78) |
10 |
28 |
2 |
III/IV |
Chemotherapy |
Y |
|
Exp |
46 |
25 |
21 |
61.7 (42-77) |
11 |
34 |
1 |
Combination |
|||
|
Liu201624 |
Con |
30 |
26 |
4 |
53 |
- |
- |
- |
III/IV |
Chemotherapy |
Y |
|
Exp |
30 |
24 |
6 |
55 |
- |
- |
- |
Combination |
|||
|
LiuY201625 |
Con |
40 |
25 |
15 |
59 (35-78) |
22 |
12 |
6 |
III/IV |
Chemotherapy |
Y |
|
Exp |
40 |
27 |
13 |
59 (41-83) |
24 |
11 |
5 |
Combination |
|||
|
Wang201626 |
Con |
60 |
37 |
23 |
63 |
- |
- |
- |
III |
TCM |
Y |
|
Exp |
60 |
38 |
22 |
62 |
- |
- |
- |
Combination |
|||
|
Zhao201627 |
Con |
43 |
29 |
14 |
33-68 |
15 |
20 |
8 |
III |
Chemotherapy |
Y |
|
Exp |
43 |
30 |
13 |
32-70 |
16 |
21 |
6 |
Combination |
|||
|
LiuLJ201828 |
Con |
41 |
27 |
14 |
65.7 (60-79) |
- |
- |
- |
III |
Chemotherapy |
Y |
|
Exp |
42 |
29 |
13 |
65.8 (60-78) |
- |
- |
- |
Combination |
|||
|
Sun201829 |
Con |
35 |
27 |
8 |
60.3 (34-78) |
25 |
10 |
0 |
III |
Chemotherapy |
Y |
|
Exp |
35 |
25 |
10 |
59.2 (35-81) |
23 |
12 |
0 |
Combination |
|||
|
Yang201830 |
Con |
33 |
19 |
14 |
56.4 (40-70) |
16 |
12 |
5 |
Early |
Chemotherapy |
Y |
|
Exp |
34 |
21 |
13 |
55.8 (41-69) |
14 |
13 |
7 |
Combination |
|||
|
Zhang201831 |
Con |
40 |
29 |
11 |
61.3 |
- |
- |
- |
- |
Chemotherapy |
Y |
|
Exp |
40 |
27 |
13 |
62.7 |
- |
- |
- |
Combination |
|||
|
Deng201932 |
Con |
47 |
26 |
21 |
61.3 (38-77) |
7 |
40 |
0 |
III/IV |
Chemotherapy |
Y |
|
Exp |
47 |
24 |
23 |
60.6 (35-78) |
10 |
37 |
0 |
Combination |
|||
|
Fu201933 |
Con |
24 |
15 |
9 |
63.2 |
14 |
10 |
0 |
I/II |
Chemotherapy |
Y |
|
Exp |
25 |
16 |
9 |
63.2 |
15 |
10 |
0 |
Combination |
|||
|
Guo201934 |
Con |
43 |
- |
- |
61 |
29 |
14 |
0 |
- |
Chemotherapy |
Y |
|
Exp |
43 |
- |
- |
62 |
30 |
13 |
0 |
Combination |
|||
|
Sun202035 |
Con |
35 |
19 |
16 |
60.2 |
8 |
20 |
7 |
III/IV |
Chemotherapy |
Y |
|
Exp |
32 |
22 |
10 |
59.4 |
8 |
19 |
5 |
Combination |
|||
|
Zhang202036 |
Con |
46 |
23 |
23 |
57.5 (28-87) |
- |
- |
- |
I/II/IIIA |
Chemotherapy |
Y |
|
Exp |
46 |
24 |
22 |
60.6 (29-89) |
- |
- |
- |
I/II/IIIA |
Combination |
||
|
Feng201237 |
Con |
30 |
18 |
12 |
63.6 |
13 |
17 |
0 |
IIIB/IV |
Chemotherapy+TCM |
Y |
|
Exp |
30 |
18 |
12 |
63.8 |
12 |
18 |
0 |
Combination |
|||
|
Yu201538 |
Con |
26 |
17 |
9 |
- |
11 |
13 |
2 |
III |
Chemotherapy |
Y |
|
Exp |
26 |
15 |
11 |
- |
9 |
16 |
1 |
Combination |
|||
|
M, male; F, female; Con, the control group; Exp, the experimental group; ADC, adenocarcinoma; SCC, Squamous cell carcinoma; PD, prescription dose; TCM, traditional Chinese medicine; Y, yes. |
|||||||||||
The pooled incidence of pneumothorax rate was 10.9% in the experimental group, which was obviously higher than in control group with RR = 4.91 (95% CI: 2.63 – 9.17, p <0.001). Then the prevalence of myelosuppression and gastrointestinal symptoms was not significantly different between two groups (Figure 5).
Publication bias and sensitivity analysis:
Sensitivity analysis was conducted by sequentially omitting one single study to assess the overall results with regard to all parameters. After the sensitivity analysis the pooled estimate of all parameters remained stable, suggesting that main outcomes were not being driven by any single study (Shown in Supplementary Figures 1-11). So, there was still good objectivity and stability for all parameters in present analysis.
In the present meta-analysis, Egger’s test showed that there was significant effect of publication bias CR (p <0.001), PD (p =0.02), ORR (p = 0.03), pneumothorax rate (p = 0.002). We used trim-and-fill method to adjust asymmetry, and the outcome did not change the major trend of the effect but reduced the effect size of some parameters including CR (adjusted RR = 1.50, 95%CI: 1.13 – 2.00, p = 0.0053), ORR (adjusted RR = 1.33, 95%CI: 1.22-1.45, p <0.001) , and pneumothorax rate ( adjusted RR = 1.96, 95%CI: 1.02-3.78, p = 0.04 ), and increased adjusted RR of PD (adjusted RR = 0.49, 95%CI: 0.36-0.68, p <0.0001). And there was no publication bias in other parameters (Shown in Supplementary Figures 12-15 available on JCPSP website).
DISCUSSION
This is the first systematic review and meta-analysis involving only RCTs and the largest sample size to look for therapeutic effects and safety of interstitial brachytherapy combined with systemic therapy in NSCLC till now, although pervious meta-analyses were reported with limitations.
Interstitial tumour implantation of low-dose-rate 125I seeds is one of the most efficient methods in controlling the cancer is malignant and overcoming clinical difficulties. By placing a radioactive source near or in the tumour, the implanted iodine-125 radioactive seeds can generate a high dose of radiation within the tumour volume to continuously destroy tumour cells, while only a small amount of healthy tissue receives therapeutic dose of radiation. Integrated results of the present study including 17 RCTs and 1315 cases revealed that the CR, PR, ORR, DCR, and 2-year OS could be improved by implantation of radioactive 125I seeds in combination with systemic therapy for NSCLC patients, who also experienced tolerable adverse events at a lower frequency.
The results of pooled analysis in present study showed that there was a significant improvement in CR and PR, and obvious decrease in SD and PD, in the combined treatment group over the control group. These results indicate that implantation of radioactive seeds can have a good local tumour control in NSCLC. The overall trend is consistent with previous studies,18,19 although the effect size of RRs are significant different among these reports.
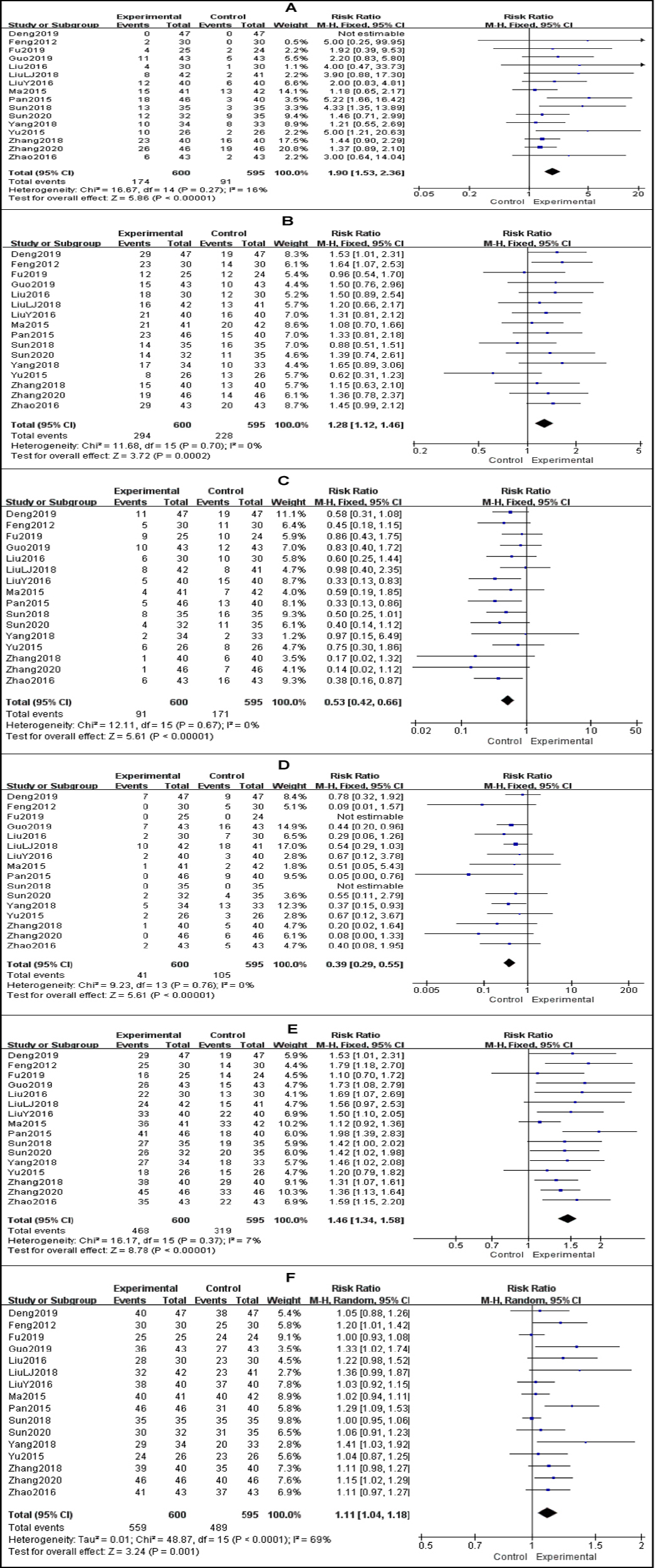 Figure 3: Meta-analysis results of the short-term efficacy between the experimental group and the control group. (A) Forest plot of studies evaluating RR of CR; (B) Forest plot of studies evaluating RR of PR; (C) Forest plot of studies evaluating RR of SD; (D) Forest plot of studies evaluating RR of PD; (E) Forest plot of studies evaluating RR of ORR; F, Forest plot of studies evaluating RR of DCR.
Figure 3: Meta-analysis results of the short-term efficacy between the experimental group and the control group. (A) Forest plot of studies evaluating RR of CR; (B) Forest plot of studies evaluating RR of PR; (C) Forest plot of studies evaluating RR of SD; (D) Forest plot of studies evaluating RR of PD; (E) Forest plot of studies evaluating RR of ORR; F, Forest plot of studies evaluating RR of DCR.
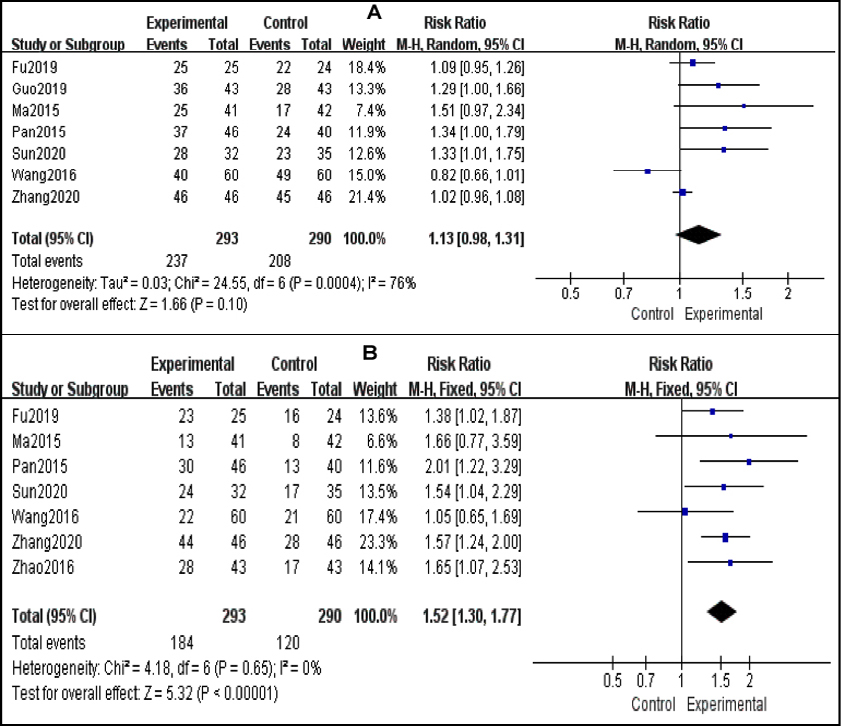 Figure 4: Meta-analysis results of overall survival between the experimental group and the control group. (A) Forest plot of studies evaluating RR of one-year OS; (B) Forest plot of studies evaluating RR of two-year OS.
Figure 4: Meta-analysis results of overall survival between the experimental group and the control group. (A) Forest plot of studies evaluating RR of one-year OS; (B) Forest plot of studies evaluating RR of two-year OS.
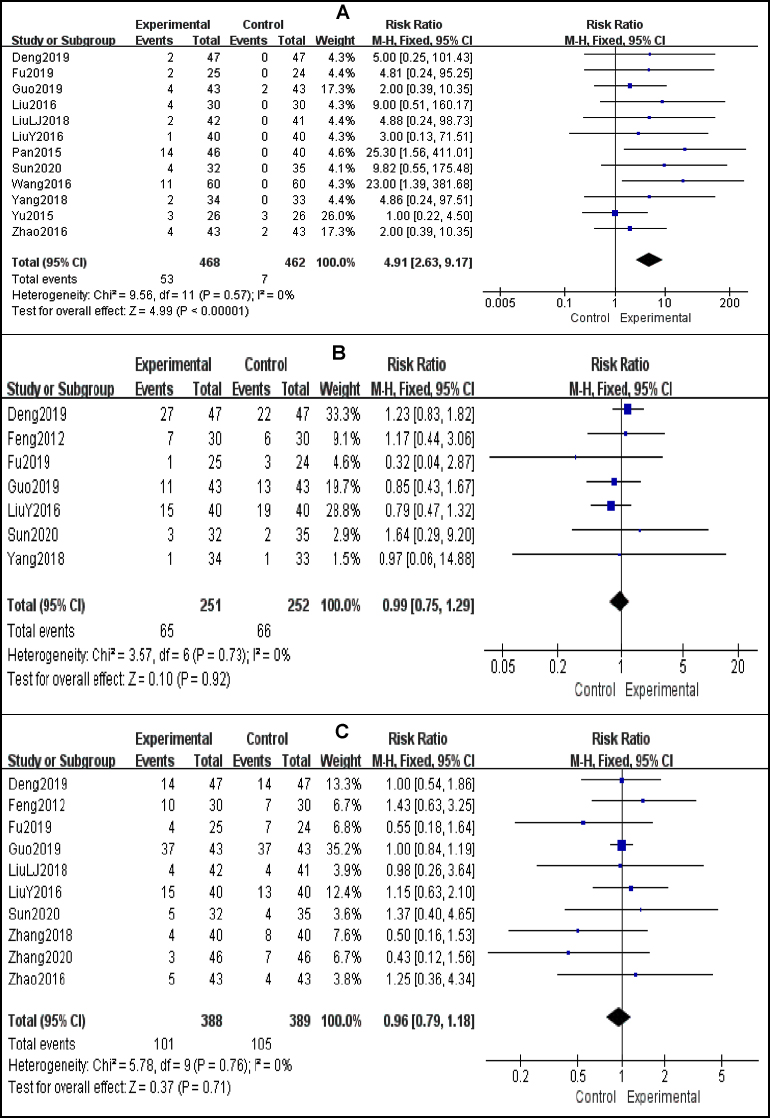 Figure 5: Meta-analysis results of complications between the experimental group and the control group. (A) Forest plot of studies evaluating RR of pneumothorax; (B) Forest plot of studies evaluating RR of myelosuppression; (C) Forest plot of studies evaluating RR of gastrointestinal symptoms.
Figure 5: Meta-analysis results of complications between the experimental group and the control group. (A) Forest plot of studies evaluating RR of pneumothorax; (B) Forest plot of studies evaluating RR of myelosuppression; (C) Forest plot of studies evaluating RR of gastrointestinal symptoms.
In Zhang,s study,16 RRs were reported as 1.84 (95% CI: 1.65-2.05) and 1.22 (95%CI: 1.14-1.29) for ORR and DCR respectively. In Qiu et al.’s study19, RRs were 3.66 (95% CI: 2.08 - 6.44, p <0.001), 1.47 (95% CI: 1.16 - 1.86, p = 0.001), 1.85 (95% CI: 1.54 - 2.22, p <0.001) and 1.19 (95% CI: 1.10 - 1.29, p <0.001) for CR, PR, ORR and DCR respectively. But we included only RCTs to achieve higher statistical reliability. It also may suggested that short-term therapeutic assessment was overvalued while mixed with non-RCTs, then it may delay the choice of further treatment in NSCLC patients.
Interstitial seed implantation has previously been shown to significantly improve OS rates in advanced-stage NSCLC patients relative to chemotherapy.39-41 To assess long-term efficacy of combination treatment with implantation of radioactive 125I seeds and systemic therapy, we assessed one-year and two-year OS rate in present meta-analysis. In another meta-analysis involving RCTs,19 it was found that there was a higher one-year OS (RR = 1.46, 95% CI: 1.12 - 1.92, p = 0.006 ) in the combined group, meanwhile, no significant difference in two-year OS ( RR = 1.30, 95% CI: 0.72 - 2.37, p = 0.39 ). The divergence may be attributed to eligible trials and different samples, and potential bias may due to a small sample in the study while mingled with non-RCTs.19
The main side effects we assessed in our meta-analysis were pneumothorax, myelosuppression and gastrointestinal symptoms. Of these, pneumothorax was the main complication of implantation, which may have been induced mainly by transpleural puncture. So, the risk of pneumothorax was higher for patients with seed implantation, compared with those without invasive treatment. The pooled incidence of pneumothorax detected in present study was 10.9%, which was in line with reports of rates in previous studies (14.8%-25.3%).13,42,43
Myelosuppression including leucopenia, anemia and thrombocytopenia, and gastrointestinal symptoms including nausea, vomiting and diarrhea are common consequences of chemotherapy and radiotherapy. They were found in patients in experimental group as well as control group. Similar to previous reports, there was no significant difference between two groups.16,18,19 Interstitial seed implantation does not induce an increase in the incidence of myelosuppression and gastrointestinal adverse reactions, which were mainly caused by systemic chemotherapy in included trials. In the other hand, brachytherapy is associated with fewer side effects as compared to external beam radiotherapy.
This study has several limitations. First, considering the characteristic of the minimally invasive treatment,44 it is difficult to completely random allocation with blind method, which may lead to the emergence of bias. Second, the duration of short-term evaluation was not consistent, which may induce report bias of clinical responses. Third, various chemotherapy regimens with different dose were used in the trials, which might affect the outcome. Fourth, the prescription dose of seed-brachytherapy was not the same in the studies. Fifth, the sample size was small, thereby potentially adding more selection bias. Hence, more high-quality, multiple-center and large-sample clinical RCTs should be performed to ensure the clinical efficacy and safety and to support the wide-spread use of iodine 125 implant in combination with systemic therapy in NSCLC.
CONCLUSION
These data indicate that combination treatment with iodine 125 implant implantation of radioactive iodine-125 seeds and systemic therapy can significantly improve clinical response and prolong two-year OS in NSCLC patients without increasing the incidence of myelosuppression and gastrointestinal symptoms, except pneumothorax.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
HL, LZ: Design of the work.
HL, LZ, WL, HZ: Data extraction.
LZ, HZ: Quality assessment of data.
HL, WF: Draft of manuscript.
WF: Final approval of the version.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Huckle A, Al-Qaisieh B, Bownes P. Methods of verifying the output of the treatment planning system used for high dose rate (HDR) prostate brachytherapy. Radiother Oncol 2012; 103(2):261-5. doi: 10.1016/j.radonc.2011.09.022.
- Junjie W. The concept of stereotactic ablation brachytherapy and practice. Chin J Radiol Med Prot 2020; 40(3):173-7.
- Fleischman EH, Kagan AR, Streeter OE, Tyrell J, Wollin M, Leagre CA, et al. Iodine125 interstitial brachytherapy in the treatment of carcinoma of the lung. J Surg Oncol 1992; 49(1):25-28. doi: 10.1002/jso.2930490107.
- Martinez-Monge R, Pagola M, Vivas I, Lopez-Picazo JM. CT-guided permanent brachytherapy for patients with medically inoperable early-stage non-small cell lung cancer (NSCLC). Lung Cancer 2008; 61(2):209-13. doi: 10.1016/j.lungcan.2007.12.016.
- Li J, Yu M, Xiao Y, et al. Computed tomography fluoroscopy-guided percutaneous (125)I seed implantation for safe, effective and real-time monitoring radiotherapy of inoperable stage T1-3N0M0 non-small-cell lung cancer. Mol Clin Oncol 2013; 1(6):1019-24. doi: 10.3892/mco.2013.171.
- Zhang T, Lu M, Peng S, Zhang W, Yang G, Liu Z, et al. CT-guided implantation of radioactive 125I seed in advanced non-small-cell lung cancer after failure of first-line chemotherapy. J Cancer Res Clin Oncol 2014; 140(8): 1383-90. doi: 10.1007/s00432-014-1655-x.
- Huo X, Wang H, Yang J, Li X, Yan W, Huo B, et al. Effectiveness and safety of CT-guided (125) I seed brachytherapy for postoperative locoregional recurrence in patients with non-small cell lung cancer. Brachytherapy 2016; 15(3):370-80. doi: 10.1016/j.brachy.2016.02.001.
- Peters N, Wieners G, Pech M, Hengst S, Ruhl R, Streitparth F, et al. CT-guided interstitial brachytherapy of primary and secondary lung malignancies: Results of a prospective phase II trial. Strahlenther Onkol 2008; 184(6):296-301. doi: 10.1007/s00066-008-1718-5.
- Manning MA, Zwicker RD, Arthur DW, Arnfield M. Biologic treatment planning for high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys 2001; 49(3):839-45. doi: 10. 1016/s0360-3016(00)01453-x.
- Chen H, Bao Y, Yu L, Jia R, Cheng W, Shao C. Comparison of cellular damage response to low-dose-rate 125I seed irradiation and high-dose-rate gamma irradiation in human lung cancer cells. Brachytherapy 2012; 11(2):149-56. doi: 10.1016/j.brachy.2011.05.002.
- Yan W, Huo X, Wang H, Huo B, Guo Y, Dong H, et al. (125)I inhibited the NSCLC both in vivo and in vitro. Int J Clin Exp Pathol 2018; 11(3):1265-72.
- Wu C, Li B, Sun G, Peng C, Xiang D. Efficacy and safety of iodine-125 brachytherapy combined with chemotherapy in the treatment of advanced NSCLC in the elderly. Onco Targets Ther 2018; 11:6617-24. doi: 10.2147/OTT.S17 4457.
- Li H, Duan Z, Zhao C, Fang W, Jia Y, Li X, et al. Combination of brachytherapy with iodine-125 seeds and systemic chemotherapy versus systemic chemotherapy alone for synchronous extracranial oligometastatic non-small cell lung cancer. Cancer Manag Res 2020; 12:8209-20. doi: 10.2147/CMAR.S267694.
- Yue TH, Xing W. (125)I seed brachytherapy combined with single-agent chemotherapy in the treatment of non-small-cell lung cancer in the elderly: A Valuable solution. Onco Targets Ther 2020; 13:10581-91. doi: 10.2147/OTT.S272898.
- Huo X, Huo B, Wang H, Wang L, Cao Q, Zheng G, et al. Implantation of computed tomography-guided Iodine-125 seeds in combination with chemotherapy for the treatment of stage III non-small cell lung cancer. J Contemp Brachytherapy 2017; 9(6):527-34. doi: 10.5114/jcb.2017. 72605.
- Zhang W, Li J, Li R, Zhang Y, Han M, Ma W. Efficacy and safety of iodine-125 radioactive seeds brachytherapy for advanced non-small cell lung cancer-A meta-analysis. Brachytherapy 2018; 17(2):439-48. doi: 10.1016/j. brachy.2017.11.015.
- Jiang G, Li Z, Ding A, Zhou F, Jiao W, Tang D, et al. Computed tomography-guided iodine-125 interstitial implantation as an alternative treatment option for lung cancer. Indian J Cancer 2015; 51 Suppl 2:e9-12. doi: 10.4103/0019-509X.151999.
- Wu H, Li L, Yang J, Yang ZM. Radioactive seeds insertion with chemotherapy for advanced non-small-cell lung cancer: A meta-analysis. Clin Respir J 2020; 15(2):187-95. doi: 10.1111/crj.13283.00.
- Qiu H, Ji J, Shao Z,. The efficacy and safety of iodine-125 brachytherapy combined with chemotherapy in treatment of advanced lung cancer: A Meta-Analysis. J Coll Physicians Surg Pak 2017; 27(4):237-45.
- Zhang S, Zheng Y, Yu P, Yu F, Zhang Q, Lv Y, et al. The combined treatment of CT-guided percutaneous 125I seed implantation and chemotherapy for non-small-cell lung cancer. J Cancer Res Clin Oncol 2011; 137(12):1813-22. oi: 10.1007/s00432-011-1048-3.
- Zhang FJ, Li CX, Wu PH, Wu YX, Jiao DC, Liu J, et al. CT guided radioactive 125I seed implantation in treating localised advanced pulmonary carcinoma. Zhonghua Yi Xue Za Zhi 2007; 87(46):3272-5.
- Yaoxian M. Short-outcome of advanced lung cancer patients received brachytherapy with implantation of radioactive 125I seeds plus chemotherpay. J Medical Forum 2015; 36(5):104-5.
- Pan L, Qian J, Li F, Wang C, Hu Y, Ou J. Efficacy of 125 I particle implantation combined with chemotherapy on non-small cell lung cancer. J Clin Pulmonary Medicine 2015; 20(5):832-4.
- Liu L, Chen J, Wang G, Zhu Z, Zhang H. Efficacy of 125 I particle implantation combined with gemcitabine and cisplatin on squamous non-small cell lung cancer. China J Pharmaceutical Economics 2016; 1:51-2.
- Liu Y, Li H, Luo Z, Zhang Z. Clinical efficiency of 125- Iodine seeds implantation on lung cancer patients. Medical recapitulate 2016; 22(11):2203-6.
- Wang Y. Clinical Application of 125I Seed implantation to locally advanced and advanced non-small cell lung cancer. Soochow University 2016.
- Zhao Z, Zhao L, Wei D, Zhao C. Clinical observation of curative effect on non-small cell lung cancer received implantation of radioactive 125I seeds and chemotherapy. Chinese J Clinical Rational Drug Use 2016; 9(9B):55-6.
- Liu L, Guo Z, Bai Y, Hu M. Clinical efficiency of 125I seeds implantation on old patients with advanced non-small cell lung cancer. J Inner Mongolia Medical University 2018; 40(5):481-7.
- Sun S. Clinical efficiency of CT-guided percutaneous implantation of radioactive 125I seeds on stage III non-small cell lung cancer. J Aerospace Medicine 2018; 29(5):582-600.
- Yang J. Application effect of CT-guided percutaneous implantation of iodine 125 particle combined with GC chemotherapy on early stage non-small cell lung cancer. Chinese J Practical Medicine 2018; 45(5):73-5.
- Zhang H, Chen S, Li X, Bai Z. Effect observation of iodine 125 radioactive particles implantation therapy on non-small cell lung cancer. China Prac Med 2018; 13(34):1-3.
- Deng A, Chen J, Song J, Li X, Xu F. Effect of radioactivee 125I seeds implantation combined with GP regimen in the treatment of advanced non-small cell lung cancer. J Clinical Experimental Medicine 2019; 18(7):723-6.
- Fu W, Chen S, Zhou Q. Clinical study of radioactive particles in the treatment of inoperable early-stage non-small cell lung cancer. J Practical Radiology 2019; 35(8):1311-8.
- Guo G, Song B, Zhu C, Xiao J. Ultrasound bronchoscopic 125I seed implantation combined with systemic chemotherapy for advanced non-small cell lung carcinoma complicated by large airway stenosis: Analysis of clinical effect. J Intervent Radiology 2019; 28(6):542-6.
- Sun J, Chen S, Chen W. Short term and long term efficacy of radioactive iodine 125 seed implantation in the treatment of local non-small cell lung cancer. Chinese J Lung Disease(electronic edition) 2020; 13(5):597-601.
- Zhang N. Effect of iodine-125 radioparticle implantation therapy on quality of life in lung cancer patients with chemotherapy. J Clinical Medicine Practice 2020; 24(6): 20-3.
- Feng Y, Xiao YY, Li SD, Lin MX, Zhang Y, Wang HM, et al. The treatment of non-small cell lung cancer by interstitial I-125 seeds implantation combined with chemotherapy and Chinese medicine. Chin J Integr Med 2012; 18(9):663-9. doi: 10.1007/s11655-012-1203-y
- Yu X, Li J, Zhong X, He J. Combination of Iodine-125 brachytherapy and chemotherapy for locally recurrent stage III non-small cell lung cancer after concurrent chemoradiotherapy. BMC Cancer 2015; 15:656. doi: 10.1186/ s12885-015-1657-3.
- Gill BS, Clump DA, Burton SA, Christie NA, Schuchert MJ, Heron DE. Salvage stereotactic body radiotherapy for locally recurrent non-small cell lung cancer after sublobar resection and i(125) vicryl mesh brachytherapy. Front Oncol 2015; 5:109. doi: 10.3389/fonc.2015.00109.
- Huang Q, Chen J, Chen Q, Lai Q, Cai S, Luo K, et al. Computed tomographic-guided iodine-125 interstitial implants for malignant thoracic tumors. Eur J Radiol 2013; 82(11):2061-6. doi: 10.1016/j.ejrad.2013.05.037.
- Mutyala S, Stewart A, Khan AJ, Cormack RA, O'Farrell D, Sugarbaker D, et al. Permanent iodine-125 interstitial planar seed brachytherapy for close or positive margins for thoracic malignancies. Int J Radiat Oncol Biol Phys 2010; 76(4):1114-20. doi: 10.1016/j.ijrobp.2009.02.067.
- Heerink WJ, de Bock GH, de Jonge GJ, Groen HJ, Vliegenthart R, Oudkerk M. Complication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur Radiol 2017; 27(1):138-48. doi: 10.1007/s00330-016-4357-8.
- Li Y, Du Y, Yang HF, Yu JH, Xu XX. CT-guided percutaneous core needle biopsy for small (Clin Radiol 2013; 68(1): e43-8. doi: 10.1016/j.crad.2012.09.008.
- Stewart A, Parashar B, Patel M, O'Farrell D, Biagioli M, Devlin P, et al. American brachytherapy society consensus guidelines for thoracic brachytherapy for lung cancer. Brachytherapy 2016; 15(1):1-11. doi: 10.1016/j.brachy. 2015.09.006.