Clinicopathological Features and Prognosis of Invasive Micropapillary Carcinoma Compared to Invasive Ductal Carcinoma-NOS: Worse or Better?
By Fugen Vardar Aker1, Erhan Ekren1, Meryem Dogan1, Gunay Gurleyik2, Eda Tanrikulu3, Basak Bala Oven4Affiliations
doi: 10.29271/jcpsp.2022.09.1196ABSTRACT
Objective: To evaluate whether there are differences in invasive micropapillary carcinoma (IMPC) and invasive ductal carcinoma-NOS (IDC-NOS) according to the clinicopathological features and prognosis including molecular subtypes.
Study Design: Descriptive study.
Place and Duration of Study: Department of Pathology, University of Health Sciences, Haydarpasa Numune Training and Research Hospital, Istanbul, Turkey, from 2003 to 2016.
Methodology: Operated breast cancer cases (58 IMPC + 326 IDC-NOS), with long-term follow-up findings (cases followed up until 2020), were reviewed. The cases, whose other component was only IDC-NOS, were included in the mixed IMPC group. The clinical features, including clinical presentation, treatments, and follow-up information were obtained from the patient clinical database. The IMPC cases included in the study were re-examined, and micropapillary tumour components were confirmed based on the criteria set by the World Health Organisation (WHO). The clinicopathological findings, recurrence, and survival data of both groups were compared. In addition, IDC-NOS was divided into the molecular subgroups and compared with IMPC cases in terms of 5-year overall survival (OS).
Results: There was no significant difference between the two groups for the distribution of molecular subtypes. There was a statistically significant difference among the nuclear grade, tumour size, nodal status, lymphovascular, and perineural invasion. In the first 5-year period, the OS rate for IDC-NOS and IMPC was 90.8% and 86.2% (p<0.05). The 5-year OS rate of luminal A, luminal B, HER2, triple negative (TN), and IMPC patients was 97.6%, 91.3%, 90%, 70%, and 86.2%, respectively (p<0.05). The OS rate in patients with TN and IMPC was similar which was found significantly lower than the other groups (luminal A, luminal B, and HER2). The median OS was 51.3 months and 53.9 months for the patients with TN and IMPC, respectively (p<0.001). This difference disappeared in the 10th and 15th years of follow-up.
Conclusion: The majority of the deaths in IMPC occurred within the first 5 years. The 5-year OS rates were similar in the TN and IMPC patients. The survival pattern of IMPC is parallel with TN, Therefore, clinical, therapeutic, and prognostic evaluation in IMPC can be done like TN.
Key Words: Invasive ductal carcinoma, Invasive micropapillary carcinoma, Survival.
INTRODUCTION
Invasive micropapillary carcinoma (IMPC) of the breast is characterised by a reversal of cellular polarisation and expression of transmembrane glycoprotein mucin 1 (MUC1) in the stroma-facing surface of the cells.
The IMPC can appear in a pure form but more often presents with the other variants. Its frequency varies between 0.9-2%.1,2 Although it is generally stated that IMPC is related to a poor prognosis, it has also been stated that its prognosis is not different from that of invasive ductal carcinoma-NOS (IDC-NOS) in recent years.2-6
This study‘s rationale was to gain a clearer insight into the differences between IMPC and IDC-NOS (including molecular subtypes) in terms of prognosis and clinicopathological parameters. The objective of the study was to evaluate whether there are differences in invasive micropapillary carcinoma (IMPC) and invasive ductal carcinoma-NOS (IDC-NOS) according to the clinicopathological features and prognosis including molecular subtypes.
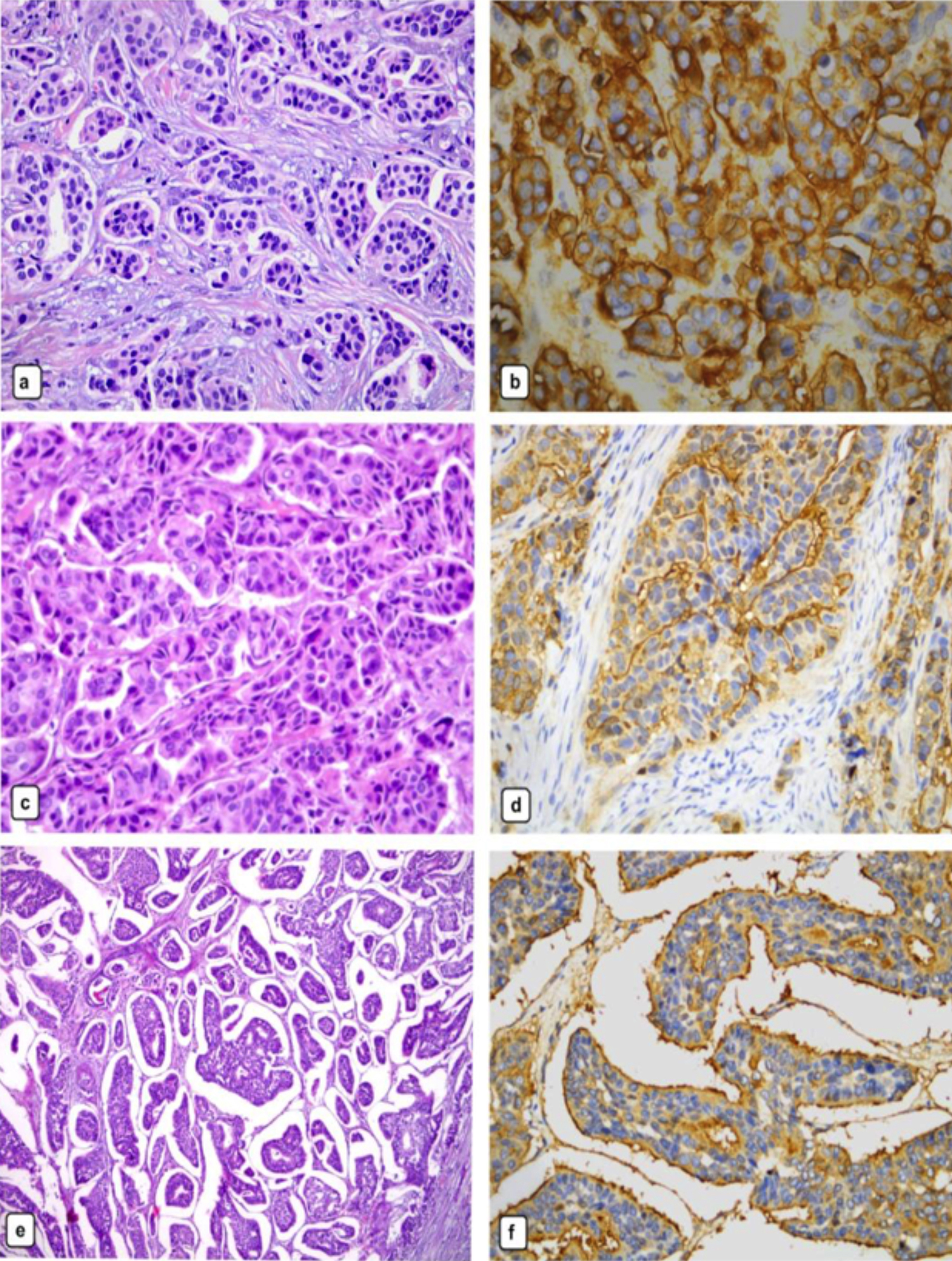 Figure 1: Invasive micropapillary carcinoma. The tumour cells have granular pink cytoplasm, high nuclear grade (a: H&EX40). The micropapillary clusters lack fibrovascular core (c: H&E X40). The characteristic sponge like appearance is due to the tumour cell clusters surrounded by the clear spaces (e:H&E X20). Immunostain for epithelial membrane antigen shows the characteristic reverse membranous staining at the outer border of the cells, facing the stroma (b,d,f: IHC staining with EMA X40).
Figure 1: Invasive micropapillary carcinoma. The tumour cells have granular pink cytoplasm, high nuclear grade (a: H&EX40). The micropapillary clusters lack fibrovascular core (c: H&E X40). The characteristic sponge like appearance is due to the tumour cell clusters surrounded by the clear spaces (e:H&E X20). Immunostain for epithelial membrane antigen shows the characteristic reverse membranous staining at the outer border of the cells, facing the stroma (b,d,f: IHC staining with EMA X40).
METHODOLOGY
This study was approved by the Institutional Review Board (771-07-2020). Data were collected during the period of study (2003–2016) from the Departments of Pathology and Medical Oncology, Haydarpasa Numune Education and Research Hospital, Istanbul, Turkey. During this period, 384 operated breast cancer cases (58 IMPC + 326 IDC-NOS), with long-term follow-up findings (cases followed up until 2020), from the series of 1244 cases registered in the department, were included in the study. The patients who did not have invasive cancer but had a history of neoadjuvant therapy or non-curative resection, or who were lost to follow-up, or those with distant metastasis, were excluded from the study. Cases, whose other component was only IDC-NOS, were included in the mixed IMPC group. The clinical features, including clinical presentation, treatments, and follow-up information were obtained from the patients‘ clinical database. The IMPC cases included in the study were re-examined and micropapillary tumour components were confirmed based on the criteria set by WHO7 (Figure 1). The clinicopathological findings, recurrence, and survival data of both groups were compared. In addition, IDC-NOS was divided into molecular subgroups and compared with the IMPC cases in terms of 5-year OS. The disease-free survival (DFS) was defined as the time from the surgery to the last follow-up or the first relapse. On the other hand, the OS was defined as the time from surgery to the last follow-up or death.
The number cruncher statistical system (NCSS), 2007 (Kaysville, Utah, USA) program was used for the statistical analysis. The descriptive statistical methods (mean, standard deviation, median, frequency, ratio, minimum, and maximum) were used when evaluating the study data.
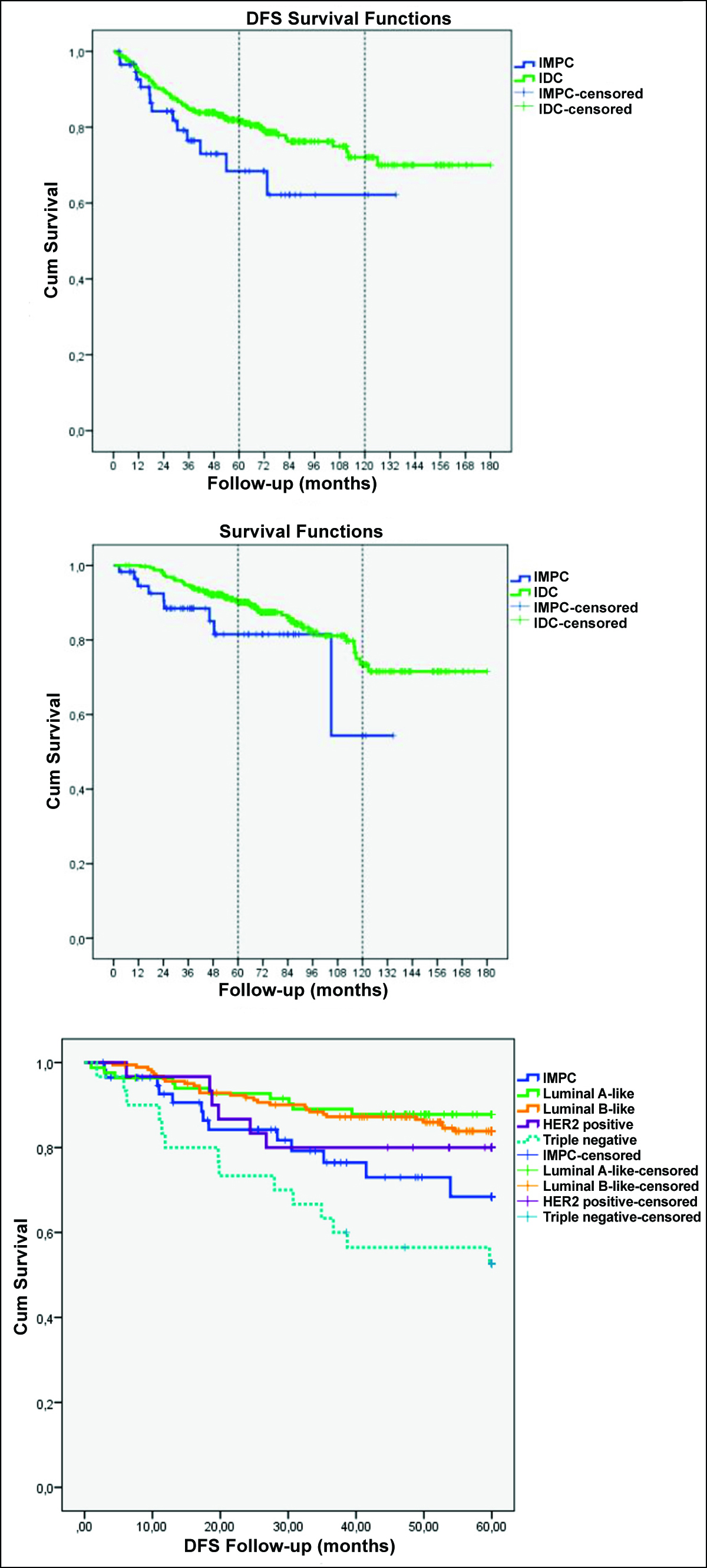 Figure 2: (a) Disease-free survival in IMPC and IDC-NOS. (b) Overall survival in IMPC and IDC-NOS. (c) Overall Survival in IMPC and molecular subgroups of IDC-NOS.
Figure 2: (a) Disease-free survival in IMPC and IDC-NOS. (b) Overall survival in IMPC and IDC-NOS. (c) Overall Survival in IMPC and molecular subgroups of IDC-NOS.
|
|
IMPC (n=58) |
IDC-NOS (n=326) |
p |
|
|
n (%) |
n (%) |
|||
|
Age (year) |
Mean±SD |
57.12±12.97 |
54.81±12.63 |
a0.201 |
|
Gender |
Female |
56 (96.6) |
323(99.1) |
b0.118 |
|
Male |
2 (3.4) |
3(0.9) |
|
|
|
Nuclear grade |
N |
(58) |
(323) |
|
|
Grade 1 |
2 (3.4) |
19 (5.9) |
c0.05 |
|
|
Grade 2 |
17 (29.3) |
143 (44.3) |
|
|
|
Grade 3 |
39 (67.2) |
161 (49.8) |
|
|
|
Histological grade |
N |
(50) |
(314) |
|
|
Grade 1 |
6 (12.0) |
49 (15.6) |
c0.798 |
|
|
Grade 2 |
19 (38.0) |
112 (35.7) |
|
|
|
Grade 3 |
25 (50.0) |
153 (48.7) |
|
|
|
pT |
N |
(57) |
(306) |
|
|
T1 |
13 (22.8) |
144 (47.1) |
c0.002† |
|
|
T2 |
34 (59.66) |
135 (44.1) |
|
|
|
T3 |
8 (14.0) |
14 (4.6) |
|
|
|
T4 |
2 (3.5) |
10 (3.3) |
|
|
|
T1mi |
0 |
3 (1.0) |
|
|
|
pN |
N0 |
19 (32.8) |
141 (43.3) |
C<0.001† |
|
N1 |
7 (12.1) |
62 (19.0) |
|
|
|
N2 |
8 (13.8) |
32 (9.8) |
|
|
|
N3 |
21 (36.2) |
25 (7.7) |
|
|
|
N4 |
0 (0) |
2 (0.6) |
|
|
|
N0 (i+) |
2 (3.4) |
10 (3.1) |
|
|
|
N1mi |
1 (1.7) |
54 (16.6) |
|
|
|
NAC |
Yes |
9 (15.5) |
35 (10.7) |
c0.292 |
|
No |
49 (84.5) |
291 (89.3) |
|
|
|
SLNB |
Yes |
20 (34.5) |
181 (55.5) |
c0.003† |
|
No |
38 (65.5) |
145 (44.5) |
|
|
|
Localisation |
N |
(57) |
(319) |
|
|
Right |
27 (47.4) |
150 (47.0) |
c0.478 |
|
|
Left |
30 (52.6) |
161 (50.5) |
|
|
|
Bilateral |
0 |
8 (2.5) |
|
|
|
Surgical procedures |
MRM |
34 (58.6) |
130 (39.9) |
b0.015* |
|
SM+SLNB |
8 (13.8) |
30 (9.2) |
|
|
|
Lumpectomy+ SLNB |
7 (12.1) |
105 (32.2) |
|
|
|
Lumpectomy +AD |
8 (13.8) |
49 (15.0) |
|
|
|
SCM+SLNB |
1 (1.7) |
7 (2.1) |
|
|
|
SCM+ AD |
0 (0) |
5 (1.5) |
|
|
|
Lymphovascular invasion |
Yes |
47 (81.0) |
137 (42.0) |
C<0.001† |
|
No |
11 (19.0) |
189 (58.0) |
|
|
|
Perineural invasion |
N |
(57) |
(326) |
|
|
Yes |
24 (42.1) |
83 (25.5) |
c0.010* |
|
|
No |
33 (57.9) |
243 (74.5) |
|
|
|
Presence of ductal carcinoma in situ |
Yes |
50 (86.2) |
253 (77.6) |
c0.139 |
|
No |
8 (13.8) |
73 (22.4) |
|
|
|
ER status |
Positive |
51 (87.9) |
250 (76.7) |
c0.055 |
|
Negative |
7 (12.1) |
76 (23.3) |
|
|
|
PR status |
Positive |
45 (77.6) |
235 (72.1) |
c0.385 |
|
Negative |
13 (22.4) |
91 (27.9) |
|
|
|
HER2 status |
Positive |
9 (15.5) |
77 (23.6) |
c0.173 |
|
Negative |
49 (85.5) |
249 (76.4) |
|
|
|
Triple Negative status |
Yes |
2 (3.4) |
30 (9.2) |
b0.198 |
|
|
No |
56 (96.6) |
296 (90.8) |
|
|
Multifocality |
Yes |
8 (13.8) |
57 (17.5) |
c0.490 |
|
No |
50 (86.2) |
269 (82.5) |
|
|
|
Mortality |
Alive |
49 (84.5) |
278 (85.3) |
c0.876 |
|
Death |
9 (15.5 |
48 (14.7) |
|
|
|
Recurrence |
Yes |
14 (24.1) |
70 (21.5) |
c0.651 |
|
No |
44 (75.9) |
256 (78.5) |
|
|
|
Molecular subtypes |
Luminal A |
18 (31.0) |
83 (25.5) |
c0.304 |
|
-Luminal B(HER2 negative) -Luminal B(HER2 positive) -HER2 positive (non-uminal) |
29 (50.0) 5 (8.6) 4 (6.9) |
136(41.7) 47 (14.4) 30 (9.2) |
|
|
|
-Triple negative |
2 (3.4) |
30 (9.2) |
|
|
|
aStudent’s t-test. bFisher’s exact Test cPearson Chi-square Test dMann-Whitney U Test. *p<0.05 †p<0.01. AD: Axillary dissection, ER: Oestrogen receptor, M: Mean, MRM: Modified radical mastectomy, NAC: Neo-adjuvant chemotherapy, PR: Progesterone receptor, SCM: Subcutaneous mastectomy, SD: Standard deviation, SLNB: Sentinel lymph node biopsy, SM: Simple mastectomy. |
||||
|
|
N |
OS (%) |
M (months) |
OS 95% CI |
p |
|
IDC-NOS Luminal A-like |
83 |
97.6 |
59.72 |
59.18-60.26 |
|
|
Luminal B-like (HER2 negative and positive group) |
183 |
91.3 |
57.72 |
59.18-60.25 |
|
|
HER2 positive (non-luminal) |
30 |
90.0 |
57.93 |
55.40-60.00 |
0.15 |
|
Triple negative |
30 |
70.0 |
51.37 |
46.27-56.47 |
|
|
IMPC |
|
|
|
|
|
|
All Molecular subtypes of IMPC group |
58 |
86.2 |
53.97 |
49.96-57.98 |
0.28 |
|
|
|
|
|
|
0.001* |
|
Kaplan-meier analysis, log rank test *p<0.01. |
|||||
The suitability of the quantitative data for normal distribution was tested by the Kolmogorov-Smirnov and Shapiro-Wilk tests and graphical evaluations. The Student's t-test was used for comparing two groups of normally distributed quantitative data, and the Mann-Whitney U test was used for two-group comparisons of non-normally distributed data. In comparison of the qualitative data, the Pearson’s chi-square and Fisher’s Exact-tests were used. The log-rank test was used for survival, analysis and Kaplan-Meier analyses were used for the molecular subgroups.
RESULTS
Fifty-eight cases with varied proportions (10%-100%) of IMPC were included in this study. Of the 58 IMPC cases, 20 patients (35%) were identified as having pure IMPC, whereas 38 (65%) had mixed IMPC. The ratio of pure type IMPC in the main series of 1244 cases was 1.6% (20/1244). The proportion of IMPC components in the IPMC group is distributed as follows: 10% in 13 cases, 20% in 4, 40% in 5, 50% in 2, 60% in 5, 70% in 3, 80% in 5, 90% in 1 and 100% in 20 cases. Three-hundred and twenty-six cases presented with IDC-NOS. There were a statistically significant difference the tumour diameter, nodal status, and resection type, in terms of whether sentinel lymph node biopsy (SLNB) was performed or not, and presence of lymphovascular and perineural invasion in between two groups (p=0.002; p<0.001; p=0.015; p = 0.003; p<0.001 and p=0.010, respectively). Although not statistically significant, there was a close relationship in terms of oestrogen receptor (ER) status and nuclear grade (p=0.055 and p=0.05). It was observed that ER-positive cases were slightly more prevalent in the IMPC group (87.9% versus 76.7%, Table I).
The recurrence was detected in 84 out of 384 (21.9%) patients. There were no differences in 15-year, 10-year, and 5-year DFS rates between tumour types (Figure 2a). In total, 327(85.2%) of the 384 patients were still alive from 2.70 to 180.07 months of median follow-up time. Eighty four point five percent of the patients with IMPC remained alive, whereas 85.3% of the patients with IDC-NOS were alive at the end of the follow-up. The 15-year and 10-year OS rates were not significantly different between the two groups. The 5-year survival rate was better among the patients with the IDC-NOS (90.8%) as compared to the patients with IMPC (86.2%), which was statistically significant (p=0.039; p<0.05, Figure 2b). During this follow-up period, a total of 9 deaths occurred in the IMPC group, 8 of them in the first 5 years. Of these cases, 4 were pure IMPC and 4 were mixed IMPC (IMPC rate: 10% in 2 cases, 50% and 70% in 1 case each).
From the perspective of the molecular subgroup; in the pure IMPC group, 1 case was in luminal A, 1 case was in the HER2 positive (non-luminal), and 2 cases were in the TN group. In the mixed IMPC group, 3 cases were in the luminal B (HER2 negative) group, and 1 case was in the luminal A group. In the IDC-NOS group, in which 30 deaths occurred, 2 cases were luminal A, 10 cases were luminal B (HER2 negative), 6 cases were luminal B (HER2 positive), 3 cases were HER2 positive (non-luminal), and 9 cases were TN. The OS of luminal A, luminal B, and HER2 in IDC-NOS group did not differ among themselves (p=0.156). The same was true for TN of IDC-NOS and IMPC (p=0.289). But the survival rate in patients with the TN of IDC-NOS and IMPC was significantly lower than in the other groups (p=0.001, Table II and Figure 2c).
DISCUSSION
Although IMPC was first described in 1980 as a specific histological type of breast cancer, it was listed as an independent subtype in the 2003 WHO classification of breast cancer.8 The reported incidence of IMPC increased at the end of the 2000s due to increased recognition of this histological subtype by the pathologists. Although recognizing the micropapillary architecture is typically not challenging, the criteria for distinguishing between the mixed and pure IMPC remains imprecise. Lastly, it has been described as a pure invasive micropapillary carcinoma, whereas >90% of the tumour consists of hollow or morula-like aggregates of cuboidal to columnar neoplastic cells.7 In this context, the mixed forms account for 2.6–7.4% of all the invasive breast carcinomas, with pure invasive micropapillary carcinoma reported to be much less frequent than mixed cases. The 1.6% rate determined for pure IMPC in this series is consistent with the literature. However, it remains controversial whether the percentage of the micropapillary component is significant for either lymph node invasion or survival outcomes. Based on the findings from most of the literature, it is recommended that any IMPC component present in a tumour is carefully evaluated and the percentage should be clearly stated in the pathology report.9-11 In this study, 4 out of the 8 deaths that occurred in the first 5 years were in the pure group and 4 in the mixed IMPC group which supports this opinion. Therefore, importance should be given to the presence of the IMPC component, rather than its emphasised percentage.
Without exception, in the most retrospective studies conducted since the identification of IMPC, many parameter are associated that can be counted as poor prognostic markers.1,8,12 These parameters can be summarised as a larger tumour diameter, frequent nodal metastasis, and lymphovascular invasion. Consistent with the previous studies, the present study also demonstrated that patients with IMPC had more pT2 and pT3 tumours and a higher rate of pN3 nodal metastases.13,14 In parallel with these findings, there was a high frequency of lymphatic vessel invasion and perineural invasion in the IMPC group. In regard to the treatment procedures, IMPC cases underwent breast conservative surgery and SLNB procedures at a lower rate than IDC-NOS cases, and similar findings have been observed in many studies.2-5 Also, IMPC does not appear to be different from IDC-NOS, when matched for the patients‘ age/gender, histological grade, location, multifocality, the presence of ductal carcinoma in situ (DCIS), immunohistochemically defined ER, progesterone receptor (PR), HER2 status, and Ki-67 proliferation index. This is particularly noteworthy in terms of ER and PR status, as most studies have reported a higher rate of ER positivity in IMPC than in the IDC-NOS comparison group.1,2,13,14 These results are in line with the present results. However, the ER rate was 87.9% in IMPC in this series. This rate is higher than the rate of ER detected in the IDC-NOS cases (76.7%). The HER2 overexpressions are reported in a variable ratio of the cases.15,16 In this study, the HER2 positivity rate was 15%. Some studies have reported 15-20 % of the cases as TN.3,4 In this series, the TN ratio is 3.4% and their rates are quite high compared to this series. Indeed, besides ER and PR positivity, the Ki-67 proliferation index was over 20% in 64.7% of the cases. In other words, most of the cases were evaluated as luminal B (58.6%). It was found that only scattered studies provided information concerning molecular subtypes. Regarding the molecular classification; IMPC mainly presented luminal B subtype in the Vingiani et al. study.15 Others reported that more cases of IMPC fall into the luminal A subtype.10,17
As early studies have indicated, IMPC is known for its distinctive clinical features like a high incidence of lymphatic vessel invasion, axillary lymph node metastasis, and a poor prognosis.11 Despite these negative prognostic parameters, IMPC cases have the DFS and OS comparable to the IDC-NOS cases.4,18 The reporting authors tried to rationalise the relationship between an unfavourable local relapse and better overall survival by noting the aggressive treatment approach that is taken in cases of breast carcinoma with lymphatic involvement. In present series, there was no difference between the two groups in terms of 5, 10 and 15 years DFS. It was seen that IMPC cases were more disadvantageous in terms of OS in the first 5 year period. Most of the deaths (89%) occurred during this period. However, this difference disappears by the 10th and 15th years of follow-up. The IMPC cases, in addition to their negative histopathological factors, showed a clinical behaviour similar to TN of IDC-NOS within the first 5 years regardless of the molecular subtypes they have within themselves, and they were differentiated from other molecular subtypes of IDC-NOS. There are lots of studies focusing on the IMPC and IDC-NOS, but few compare IMPC to TN of IDC-NOS, when both have negative prognostic factors. Chan et al. study is one of these few studies.19 According to the results of their study, IMPC and TN of IDC-NOS had similar OS. Contrary to what is known, being highly positive hormone receptors in IMPC may not contribute positively to the prognosis. This may be related to the different molecular structures of IMPC. Thus, Marchio et al. reported that high cyclin D1 expression, high proliferation rates, and MYC amplification were significantly associated with IMPC.7 According to the results of a study conducted in 2021; Sialyl-LewisX (sLeX) may be an important supplementary diagnostic marker of IMPC, and the overexpression of sLeX on the cytomembranes in the cell clusters may serve as an independent factor indicating poor prognosis in patients with IMPC.20 Probably this and similar studies may provide benefits for the patients with IMPC in the future.
The unique characteristics of IMPC behave like TN of IDC, in spite of its higher percentage of hormone receptor expression. This study has several limitations. The retrospective nature of the study and all associated inherent biases must be acknowledged. Further, meta-analysis where subgroup analysis can be done in detail or large-scale prospective studies combined with the molecular classification, including Ki-67 proliferation index and genomic expression profile analysis, are needed to verify this study results and offer more optimal treatment modalities for this rare breast carcinoma.
CONCLUSION
The majority of the deaths in IMPC occurred within the first 5 years. The 5-year OS rates were similar in TN and IMPC patients. The survival pattern of IMPC is parallel with TN. Therefore, clinical, therapeutic, and prognostic evaluation in IMPC can be done like TN.
FUNDING SOURCES:
The authors declared that there was no funding used in this study.
ETHICAL APPROVAL:
This study was approved by the Institutional Review Board of Haydarpasa Numune Training and Research Hospital (IRB No. 771-07-2020).
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
FVA: Conception of idea and supervised the findings of this work.
MD, EE: Assisted in pathological data collection.
GG, ET, BBO: Assisted in clinical and oncological data collection.
FVA, EE: Wrote the manuscript.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Siriaunkgul S, Tavassoli FA. Invasive micropapillary carcinoma of the breast. Mod Pathol 1993;6(6):660-2.
- Chen H, Wu K, Wang M, Wang F, Zhang M, Zhang P. Invasive micropapillary carcinoma of the breast has a better long-term survival than invasive ductal carcinoma of the breast in spite of its aggressive clinical presentations: A comparison based on large population database and case–control analysis. Cancer Med 2017; 6(12):2775-86. doi: 10.1002/cam4.1227.
- Yang YL, Liu BB, Zhang X, Fu L. Invasive micropapillary carcinoma of the breast: An update. Arch Pathol Lab Med 2016; 140:799-805. doi: 10.5858/arpa.2016-0040-RA.
- Wu Y, Zhang N, Yang Q. The prognosis of invasive micropapillary carcinoma compared with invasive ductal carcinoma in the breast: A meta-analysis. BMC Cancer 2017; 17(1):839. doi: 10.1186/s12885-017-3855-7.
- Hua B, Lu X, Xiao WZ, Yang X, He SR, Wang Z. [Comparison of prognosis between invasive micropapillary carcinoma and invasive ductal carcinoma of breast: a single center, retrospective case-control study] Zhonghua Wai Ke Za Zhi 2018; 56(1):56-60. [Article in Chinese] (Abs).
- Li W, Han Y, Wang C, Guo X, Shen B, Liu F, et al. Precise pathologic diagnosis and individualized treatment improve the outcomes of invasive micropapillary carcinoma of the breast: A 12-year prospective clinical study. ModPathol 2018; 31(6):956-64. doi: 10.1038/s41379-018-0024-8.
- Marchio C, Horlings HM, Vincent-Salomon A. Invasive micropapillary carcinoma. In: WHO classification of tumours editorial board, editor. WHO classification of tumours of the breast. 5th ed. International agency for research on cancer: Lyon 2019; 128-30.
- Ellis IO, Cornelisse CJ, Schnitt SJ. Invasive breast carcinomas. In: Tavassoli FA, Devilee P, editors. WHO classification of tumours pathology and genetics of tumours of the breast and female genital organs. Lyon: IARC Press 2003; 398-9.
- Gokce H, Durak MG, Akin MM, Canda T, Balci P, Ellidokuz H, et al. Invasive micropapillary carcinoma of the breast: A clinicopathologic study of 103 cases of an unusual and highly aggressive variant of breast carcinoma. Breast J 2013; 19(4):374-81. doi: 10.1111/tbj.12128.
- Kaya C, Ucak R, Bozkurt E, Omeroglu S. The impact of micropapillary component ratio on the prognosis of patients with invasive micropapillary breast carcinoma. Jinvest Surg 2020; 33(1):31-9. doi: 10.1080/08941939. 2018.1474302.
- Chen L, Fan Y, Lang RG, Guo XJ, Sun YL, Cui LF, et al. Breast carcinoma with micropapillary features: Clinicopathologic study and long-term follow-up of 100 cases. Int J Surg Pathol 2008; 16(2):155-63. doi: 10.1177/10668 96907307047.
- Nassar H. Carcinomas with micropapillary morphology: Clinical significance and current concepts. Adv Anat Pathol 2004; 11(6):297-303. doi: 10.1097/01.pap.0000138142. 26882.fe.
- Li G, Yang S, Yao J, Wang Z, Yao G, Liu M, et al. Invasive micropapillary carcinoma of the breast had poor clinical characteristics but showed no difference in prognosis compared with invasive ductal carcinoma. World J Surg Oncol 2016; 14(1):207. doi: 10.1186/s12957-016-0960-z.
- Liu Y, Huang X, Bi R, Yang W, Shao Z. Similar prognosis for invasive micropapillary breast carcinoma and pure invasive ductal carcinoma: A retrospectively matched cohort study in China. PLoS One 2014; 9(9):e106564. doi: 10.1371/journal.pone.0106564.
- Vingiani A, Maisonneuve P, Dell'orto P, Farante G, Rotmensz N, Lissidini G, et al. The clinical relevance of micropapillary carcinoma of the breast: A case-control study. Histopathol 2013; 63(2):217-24. doi: 10.1111/his. 12147.
- Marchiò C, Iravani M, Natrajan R, Lambros MB, Geyer FC, Savage K, et al. Mixed micropapillary-ductal carcinomas of the breast: A genomic and immunohistochemical analysis of morphologically distinct components. J Pathol 2009; 218(3):301-15. doi: 10.1002/path.2572.
- Shi WB, Yang LJ, Hu X, Zhou J, Zhang Q, Shao ZM. Clinico-pathological features and prognosis of invasive micropapillary carcinoma compared to invasive ductal carcinoma: A population-based study from China. PLoS One 2014; 30:9(6):e101390. doi: 10.1371/journal.pone.0101390.
- Ye F, Yu P, Li N, Yang A, Xie X, Tang H, Liu P. Prognosis of invasive micropapillary carcinoma compared with invasive ductal carcinoma in breast: A meta-analysis of PSM studies. Breast 2020; 51:11-20. doi: 10.1016/j.breast.2020.01.041.
- Chen HL, Ding A. Comparison of invasive micropapillary and triple negative invasive ductal carcinoma of the breast. Breast 2015; 24(6):723-31. doi: 10.1016/j.breast.2015.09.001.
- Song Y, Sun H, Wu K, Lyu J, Zhang J, Gu F, et al. sLex expression in invasive micropapillary breast carcinoma is associated with poor prognosis and can be combined with MUC1/EMA as a supplementary diagnostic indicator. Cancer Biol Med 2021; 18(2):477-89. doi: 10.20892/j.issn.2095-3941.2020.0422.