Clinical Significance of Methyltransferase-like 16 Expression in Epithelial Ovarian Cancer
By Changshu Li, Chun Qiao, Huafeng Ding, Yonghong LuoAffiliations
doi: 10.29271/jcpsp.2022.12.1576ABSTRACT
Objective: To detect methyltransferase-like (METTL) 16 expression in epithelial ovarian cancer (EOC) by immunohistochemistry (IHC), and its relationship with clinicohistopathological parameters and prognosis.
Study Design: Observational study.
Place and Duration of Study: Department of Gynaecology and Pathology, The First Hospital of Wannan Medical College (Yijishan Hospital of Wannan Medical College), from February to June 2022.
Methodology: METTL16 expression in 115 EOC patients was evaluated by IHC. According to the immunoreactive score (IRS), scores <6 represented low expressions and ≥6 high expressions. Clinicopathologic data and follow-up information were collected for statistical evaluation.
Results: METTL16 expression decreased in EOC (p = 0.001) and affected the poor prognosis of EOC patients. Low METTL16 patients expression had significantly higher frequencies of advanced FIGO stage, low grade, more lymph node metastasis, high CA125 levels, bilateral disease, distant metastasis, and high frequency of neural/vascular invasion compared to high METTL16 patients (p ≤0.001, <0.001, <0.001, 0.017, 0.027, <0.001, and 0.010, respectively). The survival analysis showed that the overall survival (p <0.0001) as well as the disease-free survival (p <0.0001) were remarkably shorter in low METTL16 patients compared to high METTL16 patients, suggesting worse survival.
Conclusion: There was a clear association between the expression of METTL16, poor prognostic factors, and lower survival of EOC patients, suggesting that it might exert a vital effect on the malignant progression / prognosis of EOC.
Key Words: Epithelial ovarian cancer (EOC), METTL16, Immunohistochemistry, Prognosis.
INTRODUCTION
EOC represents 80 to 90% of ovarian cancer (OC) cases, which is the first cause of death among gynaecological malignancies worldwide.1 The standard first-line treatment regimen for EOC is maximal cytoreductive surgery and platinum-based chemotherapy.2 The efficacy of advanced EOC has recently improved with the progress of cytoreductive surgery and the development of chemotherapy regimens. However, the primary or secondary chemotherapy resistance of EOC often leads to failure, causing recurrence and metastasis and representing an overall cure rate of only 30%.2 Therefore, identifying key biomarkers and prognostic indicators involved in the malignant progression of EOC has important clinical implications.
Methyltransferase-like protein 16 (METTL16) is a human RNA N6-methyltransferase that can interact with rRNA, non-coding RNAs (ncRNAs), lncRNAs, and other mRNAs.3 Increasing evidence has demonstrated that N6-methyladenosine (m6A) methylase participates in various life activities such as metabolism, gene expression, and immune response by modifying RNAs, exerting a pivotal effect on tumourigenesis, metastasis, as well as drug resistance, and is closely related to tumour prognosis.4 Additionally, m6A methylation modification of methylases, such as METTL3, ALKBH5, and IGF2BP1, promotes the occurrence and progression of EOC and might become a new locus and prognostic molecular marker for combined therapy of OC.5-7
Loss of METTL16 is also related to poor overall survival (OS) in patients with endocrine system tumours.8 Moreover, increased expression of METTL16 promotes cancer cell proliferation and division in gastric cancer.9 However, low expression of METTL16 in hepatocellular carcinoma is associated with the activation of multiple metabolic pathways, suggesting a worse prognosis.10 In oxidatively stressed lung fibroblasts, low expression of METTL16 promotes acute premature cell senescence.11 In pancreatic cancer, METTL16 is involved in the immune infiltration response of tumours.12
The ovary is an important hormone-secreting organ in the female reproductive system, and EOC is one of the most common and dangerous cancers in women. Based on the above studies on METTL16 in endocrine system diseases and cancer, the authors found that METTL16 was significantly underexpressed in OC by TCGA database analysis. It was speculated whether METTL16 plays a vital role in EOC and has any association between the protein levels of METTL16 and tumour malignancy, clinical features, and survival in EOC patients which may have a clinical value of changes in METTL16 protein expression.
METHODOLOGY
Malignant (n = 115) and normal (n = 20) ovarian tissues from patients who underwent cytoreductive surgery were collected, from February 2015 to December 2017 in the First Hospital of Wannan Medical College.
The inclusion criteria were the patients with initial confirmed diagnosis of OC, who underwent initial optimal resection of the tumour. Their postoperative specimens were pathologically confirmed as primary EOC or normal ovarian tissue. The patients with complete background data and those who received no neoadjuvant therapy before the surgery and were not diagnosed with other cancers, were included. The exclusion criteria were other malignant tumours; severe liver, kidney, lung, gastrointestinal tract, and heart diseases; immunodeficiency and coagulopathy; diabetes and other chronic metabolic disorders; and exposure to radiotherapy or chemotherapy before surgery. Postoperative adjuvant therapies were administered based on standard schedules and doses. Follow-up information was collected for all those meeting the inclusion criteria for five years until the recurrence occurred or the patient died. Follow-up included gynecologic pelvic examination, testing for anticancer 125 or other tumour markers, and computed tomography (CT) or ultrasonography of the chest, abdomen, or pelvis. The Ethics Committee of the First Hospital of Wannan Medical College approved the protocol of this study.
Immunohistochemistry (IHC) was adopted to determine the protein expression of METTL16 in resected EOC along with normal ovarian tissues after surgery. Tissue samples were stained with the peroxidase method, distributed, and incubated with rabbit anti-METTL16 primary antibody (Proteintech, 19924-1-AP, 1:500), secondary antibody (Beyotime, A0208, 1:50), then developed using diaminobenzidine (DAB) for 3 minutes. Next, samples were immersed in a hematoxylin bath at room temperature and stained for nuclei for 2.5 minutes.
Sections were scored using a light microscope, with five random fields photographed at 400×. Since METTL16 was present in the nucleus and cytoplasm, the evaluation criteria were based on brownish-yellow granular staining in tumour nuclei and the cytoplasm as positive. According to the immunoreactive score (IRS) developed by Remmele et al.,13 tissue sections were scored by light microscope according to the staining degree (0-3 for negative staining, yellowish, light brown, and dark brown respectively) and positivity extent (0-4 for 0, 1-25, 26-50, 51-75, and 76-100% respectively). A total score of 0-3 indicated considered negative (-), 3-6 indicated weak (+), 6-8 indicated moderate (++), and 9-12 indicated strong (+++). Total scores <6 represented low expressions and ≥6 high expressions. Two senior pathologists judged the results independently, and a new pathologist resolved possible objections.
All statistical analyses were performed using SPSS software (v. 26.0). Descriptive data are presented as frequencies (percentages) and means ± standard deviations (SDs). Qualitative variables were analysed by the χ2 or Fisher exact test to compare high and low METTL16 groups. The Kaplan-Meier method was applied in the evaluation of the associations between the overall survival time (OS)/disease-free survival time (DFS) and the expression of METTL16 and draw survival curves. Multivariate Cox regression analysis was carried out to explore the prognostic factors. A value of p <0.05 was considered statistically significant.
RESULTS
Tumour tissue sections from 115 EOC patients were analysed for METTL16 expression by IHC (Figure 1). Low METTL16 expression was detected in 73 (63.5%) EOC tissue samples, while 42 cases (36.5%) showed high METTL16 expression (p = 0.001). Among the 20 normal ovarian tissues, METTL16 expression was elevated in 17 cases (85.0%) and decreased in 3 cases (15%). The mean age of patients was 53.71 ± 8.66 years (range: 35-78 years). Among the 115 EOC cases, the major histological type was serous adenocarcinoma (74.8%, 86 cases), followed by mucinous adenocarcinoma (10.4%, 12 cases), clear cell carcinoma (10.4%, 12 cases), and endometrioid carcinoma (4.3%, 5 cases). Additionally, 36 (31.3%) patients had stage I, 25 (21.7%) had stage II, 35 (30.4%) had stage III, and 19 (16.5%) had stage IV.
The χ2 analysis revealed that low expression of METTL16 had significantly higher frequencies of advanced FIGO stage (p <0.001), more lymph node metastasis (p <0.001), higher grade (p <0.001), higher CA125 levels (p = 0.017), bilateral lesions (p = 0.027), distant metastasis (p <0.001), and higher probability of nerve/vessel invasion (p = 0.010). However, there was no difference found for age, tumour size, as well as histologic subgroups (p >0.05). The clinicopathological information of EOC patients is presented in Table I.
The median follow-up time for EOC patients was 26 months (range: 6-60 months), and 61 patients died during follow-up. The Kaplan-Meier analysis showed that low METTL16 patients had shorter OS than high METTL16 patients (p <0.0001, Figure 2). A similar influence of low METTL16 was observed for DFS (p <0.0001, Figure 2). Furthermore, we have performed multivariate analyses to evaluate the prognostic factors. METTL16 levels as well as FIGO stage were independent prognostic indicators for OS & DFS (p <0.05, Table II). Altogether, these results proved that the low expression of METTL16 was associated with poorer DFS & OS with statistical significance.
Table I: Correlation analysis between clinical data and expression of METTL16.|
Variable |
METTL16 expression level |
Total |
p-value* |
|
|
|
High, n (%) |
Low, n (%) |
|
|
|
EOC group |
42(36.5) |
73(63.5) |
115 |
0.001 |
|
Normal |
17(85) |
3(15) |
20 |
|
|
Total |
59(43.7) |
76(56.3) |
135 |
|
|
Age, years |
|
|
|
|
|
<50 |
17(14.8) |
26(22.6) |
43 |
0.604 |
|
≥50 |
25(21.7) |
47(40.9) |
72 |
|
|
FIGO stage |
|
|
|
|
|
I |
24(20.9) |
12(10.4) |
36 |
<0.001 |
|
II |
11(9.6) |
14(12.2) |
25 |
|
|
III |
7(6.1) |
28(24.3) |
35 |
|
|
IV |
0(0) |
19(16.5) |
19 |
|
|
Tumour size, cm |
|
|
|
|
|
<5 |
7(6.1) |
11(9.6) |
18 |
0.820 |
|
≥5 |
35(30.4) |
62(53.9) |
97 |
|
|
Lymph node metastasis |
|
|
|
|
|
Yes |
6(5.2) |
46(40) |
52 |
<0.001 |
|
No |
36(31.3) |
27(23.5) |
63 |
|
|
Grade |
|
|
|
|
|
G1 |
22(19.1) |
13(11.3) |
35 |
<0.001 |
|
G2+G3 |
20(17.4) |
60(52.2) |
80 |
|
|
Histologic subgroups |
|
|
|
|
|
Serous |
28(24.3) |
58(50.4) |
86 |
0.067 |
|
Mucinous |
3(2.6) |
9(7.8) |
12 |
|
|
Endometrioid |
3(2.6) |
2(1.7) |
5 |
|
|
Clear cell |
8(7) |
4(3.5) |
12 |
|
|
CA125 level (IU/ml) |
|
|
|
|
|
<489 |
27(23.5) |
30(26.1) |
57 |
0.017 |
|
≥489 |
15(13) |
43(37.4) |
58 |
|
|
Bilateral lesions |
|
|
|
|
|
Yes |
20(17.4) |
50(43.5) |
45 |
0.027 |
|
No |
22(19.1) |
23(20) |
70 |
|
|
Distant metastasis |
|
|
|
|
|
Yes |
6(5.2) |
41(35.7) |
47 |
<0.001 |
|
No |
36(31.3) |
32(27.8) |
68 |
|
|
Nerve or vessel involvement |
|
|
|
|
|
Yes |
12(10.4) |
39(33.9) |
51 |
0.010 |
|
No |
30(26.1) |
34(29.6) |
64 |
|
Table II: Multivariate analysis of OS and DFS by Cox regression model.
|
Variable |
Overall survival |
Disease-free survival |
||||
|
P |
HR |
95% CI |
P |
HR |
95% CI |
|
|
FIGO stage |
0.005 |
2.043 |
1.248-3.344 |
<0.001 |
2.457 |
1.505-4.011 |
|
Grade |
0.545 |
0.805 |
0.400-1.622 |
0.473 |
0.769 |
0.375-1.578 |
|
Distant metastasis |
0.211 |
1.765 |
0.725-4.293 |
0.335 |
1.536 |
0.641-3.681 |
|
METTL16 expression level |
0.003 |
3.312 |
1.497-7.326 |
0.003 |
3.316 |
1.487-7.391 |
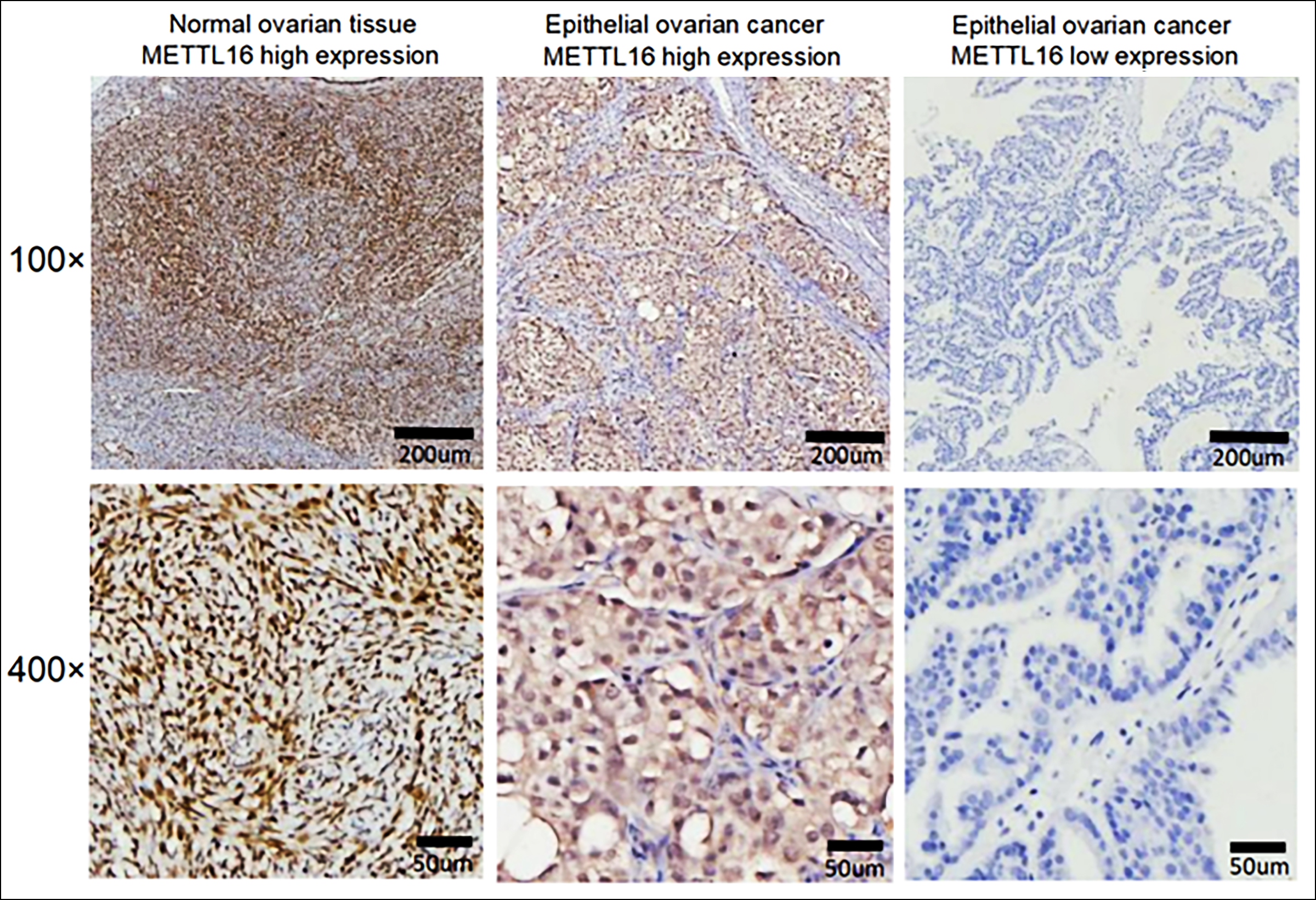 Figure 1: Expression of METTL16 by IHC in normal ovarian and EOC tissues.
Figure 1: Expression of METTL16 by IHC in normal ovarian and EOC tissues.
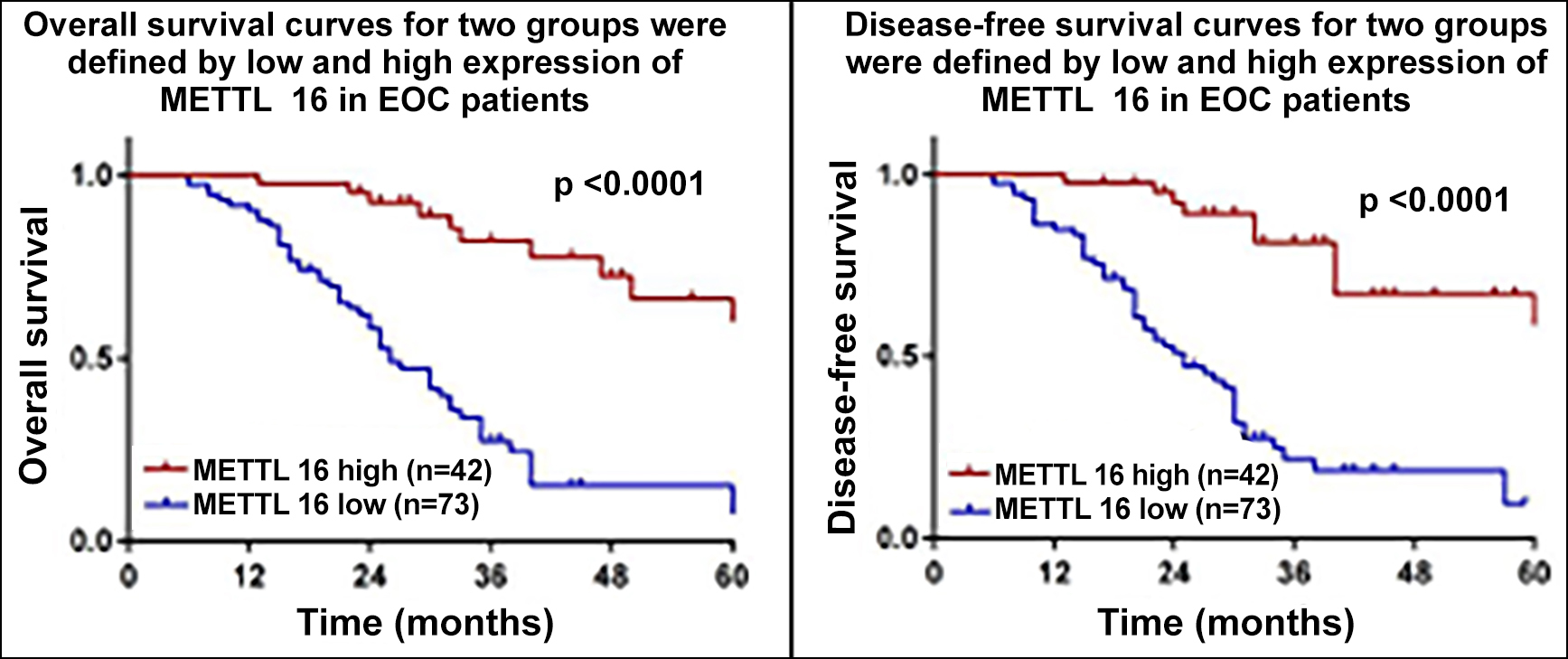 Figure 2: Correlation between the expression of METTL16 and survival of EOC patients.
Figure 2: Correlation between the expression of METTL16 and survival of EOC patients.
DISCUSSION
OC has been considered the most lethal malignant tumour in gynaecological cancers. Recurrence, metastasis, as well as therapy resistance remain critical causes of death from advanced OC. The lack of effective predictive markers for OC malignant progression and prognosis poses a great challenge in predicting its clinical outcomes. Moreover, m6A methylation has become a research hotspot. Abnormal m6A modifications play a vital role in the development of various tumours.14 Many m6A RNA methylases affect OC development and prognosis, including FTO, ALKBH5, METTL3, METTL14, RBM15, and YTHDF1.15-17 However, the absence of METTL16 in OC has never been deeply explored. Thus, we evaluated the expression of METTL16 in EOC tissues by IHC and explored its potential clinical significance.
Herein, the authors have found that METTL16 was mainly distributed in the nucleus and cytoplasm of EOC cells. Compared to normal ovarian tissues, the protein levels of METTL16 were notably lower in EOC samples (p = 0.001). Besides, the expression level of METTL16 was significantly linked to poor prognostic factors in EOC. Recent studies have shown that METTL16 acts in the nucleus as an m6A writer upon mRNAs, allowing it to undergo methylation modification. In the cytosol, METTL16 enhances the translation of various mRNAs by promoting the assembly of translation initiation complexes.18 METTL16 also exerts an effect on cancer by regulating the proliferation, division, as well as apoptosis of cells.19,20 In OC, low METTL16 expression mostly appears in stage III-IV and patients over 60 years and suggests a poor prognosis.15 In the present study, low METTL16 patients had a more advanced FIGO stage and were at greater risk of distant metastasis and invasion (p <0.001). Thus, the decrease of METTL16 might promote the invasion and migration of tumour cells, leading to cancer progression. METTL16 can also promote cell proliferation of gastric cancer via upregulating the expression level of cyclin D1.9 However, the authors have not found differences regarding the expression of METTL16 and tumour size (p >0.05), whereas it was remarkably related to the metastasis of lymph nodes (p <0.001). The association of CA125 with METTL16 was also investigated. Surprisingly, the levels of METTL16 decreased with higher levels of CA125. CA125 is the most investigated and validated biomarker in OC and a well-established marker for EOC diagnosis and patient follow-up. In the future, we shall detect the expression of METTL16 in the serum of patients to understand its predictive and diagnostic value in EOC.
The Cox multiple regression analysis showed that the FIGO stage and low METTL16 expression were risk factors affecting EOC patients (p <0.05). Additionally, the OS and DFS were remarkably shorter in low METTL16 patients compared to high METTL16 patients. These results suggested that lower METTL16 patients had poor survival. In colorectal cancer, mutations in key METTL16 residues (R200Q/G110C) have been closely related to poor prognosis.21 Meanwhile, METTL16 was found to be linked to the prognosis of EOC. Hence, we have hypothesized that mutations in METTL16 are also present in EOC development and progression. Moreover, methylation modification of METTL16 is mainly focused on three ncRNAs (U6 snRNA, MALAT1, and XIST) and one mRNA (MAT2A).19 In intervertebral disc degeneration, m6A modification of MAT2A pre-mRNA by METTL16 exacerbates apoptosis of nucleus pulposus cells22 However, the lncRNAs MALAT1 and XIST can play cancer-promoting and tumour-suppressor roles in OC.23,24 In future, the authors intend to investigate whether METTL16 impacts EOC development through downstream-regulated target RNAs, leading to poor patient survival.
Overall, it was demonstrated that METTL16 was differentially expressed in EOC and normal ovarian tissues, and its protein levels were remarkably associated with poor prognostic factors and poor survival of patients. To some extent, METTL16 is involved in EOC development and might be helpful for the prognostic evaluation of patients. However, the current study also has some limitations. A limited sample size was included and the molecular mechanisms by which METTL16 affected the progress of EOC were not deeply explored. In subsequent studies, the authors intend to expand the sample size to determine the relevance between METTL16 and existing OC prognostic markers to provide a theoretical basis for METTL16 as a new prognostic molecule in EOC. At the same time, the mechanisms underlying how METTL16 affects the prognosis of EOC remain obscure and thus is necessary for further verification in vitro and in vivo.
CONCLUSION
Expression of METTL16 was downregulated in EOC and associated with poor prognostic factors and lower survival of EOC patients. These results suggested that METTL16 might exert a pivotal effect on the malignant progression / poor prognosis in patients with EOC.
ETHICAL APPROVAL:
This study was performed with the approval of the Ethical Committee in the First Affiliated Hospital of Wannan Medical College (Approval No. 2022/03).
PATIENTS’ CONSENT:
Informed consent were obtained from all patients or relatives before the study began.
COMPETING INTEREST:
The authors declared no competing interest.
FUNDING:
This study was supported by the Project of Technology Division of Wuhu City (No. 2021cg26) and the Teaching Quality and Teaching Reform Project of colleges and universities in Anhui Province (No. 2021jyxm1625).
AUTHORS’ CONTRIBUTION:
CL: drafting the manuscript, acquisition, analysis and interpretation of data.
CQ: Collected, analysed and interpreted data.
HD: Critical revision of important work content.
YL: Drafting, conception, and designing the study content.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70(1):7-30. doi: 10.3322/caac.21590..
- Conte C, Fagotti A, Avesani G, Trombadori C, Federico A, D'Indinosante M, et al. Update on the secondary cytoreduction in platinum-sensitive recurrent ovarian cancer: a narrative review. Ann Transl Med 2021; 9(6):510. doi: 10.21037/ atm-20-4690.
- Ruszkowska A. METTL16, Methyltransferase-Like Protein 16: Current Insights into Structure and Function. Int J Mol Sci 2021; 22(4):2176. doi: 10.3390/ijms22042176.
- Zhang B, Wu Q, Li B, Wang D, Wang L, Zhou YL. M6A regulator-mediated methylation modification patterns and tumor microenvironment infiltration characterization in gastric cancer. Mol Cancer 2020; 19(1):53. doi: 10.1186/s12943- 020-01170-0.
- Bi X, Lv X, Liu D, Guo H, Yao G, Wang L, et al. METTL3-mediated maturation of miR-126-5p promotes ovarian cancer progression via PTEN-mediated PI3K/Akt/mTOR pathway. Cancer Gene Ther 2021; 28(3-4):335-49. doi: 10.1038/ s41417-020-00222-3.
- Huang H, Wang Y, Kandpal M, Zhao G, Cardenas H, Ji Y, et al. FTO-dependent n6-methyladenosine modifications inhibit ovarian cancer stem cell self-renewal by blocking cAMP signaling. Cancer Res 2020; 80(16):3200-14. doi: 10.1158/ 0008-5472.CAN-19-4044.
- Hao L, Wang JM, Liu BQ, Yan J, Li C, Jiang JY, et al. Wang HQ. m6A-YTHDF1-mediated TRIM29 upregulation facilitates the stem cell-like phenotype of cisplatin-resistant ovarian cancer cells. Biochim Biophys Acta Mol Cell Res 2021; 1868(1):118878. doi: 10.1016/j.bbamcr.2020.118878.
- Li K, Luo H, Luo H, Zhu X. Clinical and prognostic pan-cancer analysis of m6A RNA methylation regulators in four types of endocrine system tumors. Aging (Albany NY) 2020; 12(23):23931-23944. doi: 10.18632/aging.104064.
- Wang XK, Zhang YW, Wang CM, Li B, Zhang TZ, Zhou WJ, et al. METTL16 promotes cell proliferation by up-regulating cyclin D1 expression in gastric cancer. J Cell Mol Med 2021; 25(14):6602-17. doi: 10.1111/jcmm.16664.
- Wang P, Wang X, Zheng L, Zhuang C. Gene signatures and prognostic values of m6A regulators in hepatocellular carcinoma. Front Genet 2020; 11:540186. doi: 10.3389/fgene. 2020.540186.
- Wu F, Zhang L, Lai C, Peng X, Yu S, Zhou C, et al. Dynamic alteration profile and new role of RNA m6A methylation in replicative and H2O2-induced premature senescence of human embryonic lung fibroblasts. Int J Mol Sci 2022; 23(16):9271. doi: 10.3390/ijms23169271.
- Zhang T, Sheng P, Jiang Y. m6A regulators are differently expressed and correlated with immune response of pancreatic adenocarcinoma. J Cancer Res Clin Oncol 2022. doi: 10.1007/s00432-022-04150-7.
- Remmele W, Stegner HE. Vorschlag zur einheitlichen Definition eines Immunreaktiven Score (IRS) für den immunhistochemischen Ostrogenrezeptor-Nachweis (ER-ICA) im Mammakarzinomgewebe [Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue]. Pathologe 1987; 8(3):138-40. German. PMID: 3303008.
- Zhao BS, Roundtree IA, He C. Post-transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol 2017; 18(1):31-42. doi: 10.1038/nrm.2016.132.
- Zhang C, Liu J, Guo H, Hong D, Ji J, Zhang Q, et al. m6A RNA methylation regulators were associated with the malignancy and prognosis of ovarian cancer. Bioengineered 2021; 12(1):3159-76. doi: 10.1080/21655979.2021.1946305.
- Nie S, Zhang L, Liu J, Wan Y, Jiang Y, Yang J, et al. ALKBH5-HOXA10 loop-mediated JAK2 m6A demethylation and cisplatin resistance in epithelial ovarian cancer. J Exp Clin Cancer Res 2021; 40(1):284. doi: 10.1186/s13046-021- 02088-1.
- Bi X, Lv X, Liu D, Guo H, Yao G, Wang L, et al. METTL3 promotes the initiation and metastasis of ovarian cancer by inhibiting CCNG2 expression via promoting the maturation of pri-microRNA-1246. Cell Death Discov 2021; 7(1):237. doi: 10.1038/s41420-021-00600-2.
- Su R, Dong L, Li Y, Gao M, He PC, Liu W, et al. METTL16 exerts an m6A-independent function to facilitate translation and tumorigenesis. Nat Cell Biol 2022; 24(2):205-216. doi: 10.1038/s41556-021-00835-2.
- Satterwhite ER, Mansfield KD. RNA methyltransferase METTL16: Targets and function. Wiley Interdiscip Rev RNA 2022; 13(2):e1681. doi: 10.1002/wrna.1681.
- Nance DJ, Satterwhite ER, Bhaskar B, Misra S, Carraway KR, Mansfield KD. Characterization of METTL16 as a cytoplasmic RNA binding protein. PLoS One 2020; 15(1):e0227647. doi: 10.1371/journal.pone.0227647.
- Giannakis M, Mu XJ, Shukla SA, Qian ZR, Cohen O, Nishihara R, et al. Genomic correlates of immune-cell infiltrates in colorectal carcinoma. Cell Rep 2016; 17(4):1206. doi: 10.1016/j.celrep.2016.10.009.
- Chen PB, Shi GX, Liu T, Li B, Jiang SD, Zheng XF, et al. Oxidative stress aggravates apoptosis of nucleus pulposus cells through m6A modification of MAT2A Pre-mRNA by METTL16. Oxid Med Cell Longev 2022; 2022:4036274. doi: 10.1155/ 2022/4036274.
- Mao TL, Fan MH, Dlamini N, Liu CL. LncRNA MALAT1 facilitates ovarian cancer progression through promoting chemoresistance and invasiveness in the tumor microenvironment. Int J Mol Sci 2021; 22(19):10201. doi: 10.3390/ijms221910201.
- Guo T, Yuan D, Zhang W, Zhu D, Xiao A, Mao G, et al. Upregulation of long noncoding RNA XIST has anticancer effects on ovarian cancer through sponging miR-106a. Hum Cell 2021; 34(2):579-87. doi: 10.1007/s13577-020-00469-w.