Clinical Significance of Expression of Periostin in Non-small Cell Lung Cancer
By Zhixiong Qiao1, Lan Zhang2, Jinxi He1, Wei He1Affiliations
doi: 10.29271/jcpsp.2022.09.1149ABSTRACT
Objective: To analyse the expression of serum periostin in patients with non-small cell lung cancer (NSCLC) and its clinical significance.
Study Design: Descriptive study.
Place and Duration of study: General Hospital of Ningxia Medical University, Yinchuan, China, from October 2018 to October 2019.
Methodology: Patients with NSCLC, benign lung lesions, and healthy volunteers (controls) were enrolled. Serum periostin level of all the patients with NSCLC was determined by the enzyme-linked immunosorbent assayed upon admission. The receiver operating characteristic (ROC) curve was used to evaluate the value of serum periostin predicting metastasis and recurrence.
Results: The serum periostin level in the NSCLC group (n=66) was significantly higher than that of the benign group (n=40), control group (n= 38, p<0.001), and in patients with different T and N stages in the NSCLC group (p<0.001). The serum levels of periostin in patients with metastasis and recurrence within one year were significantly higher than those without that (p<0.001). When the serum periostin level was 54.12 ng/mL, the area under the curve (AUC) predicting postoperative recurrence of NSCLC in patients was 0.739, with 69.23% sensitivity and 75.47% specificity. When the serum periostin level was 42.84 ng/mL, the AUC predicting postoperative metastasis of NSCLC in patients was 0.831, with 80.00% sensitivity and 82.93% specificity.
Conclusion: Serum periostin level is possibly related to the progression of NSCLC and exhibited certain predictive values for the prognosis of NSCLC patients. The value of periostin level predicting metastasis was greater than predicting recurrence at the studied levels.
Key Words: Non-small cell lung cancer, Periostin expression, Clinical significance.
INTRODUCTION
Lung cancer is one of the common malignant tumours of the respiratory system. Its morbidity and mortality have ranked first among all the malignant tumours, becoming a global public health problem.1,2 It can be divided into non-small cell lung cancer (NSCLC) and small cell lung cancer according to the histological characteristics of the lung cancer. NSCLC accounts for more than 80% of all the lung cancer cases, it is highly malignant and has quick progression.3,4 Due to the lack of specificity in the early manifestations, most patients are diagnosed with NSCLC at the advanced stages leading to a poor prognosis.5,6
It’s well known that accurate staging before surgery is prerequisite for the surgical treatment.7 Current diagnostic imaging techniques, such as positron emission tomography-computed tomography (PET-CT) and endoscopic ultrasound- guided transbronchial needle biopsy (EBUS-TBNA) are expensive.8,9 Additionally, although surgical resection of lung cancer can prolong the survival of the patients to a certain extent, there is still a risk of recurrence and metastasis after the surgery.10 Therefore, finding reliable diagnostic markers to achieve early-stage diagnosis has important clinical significance for improving the survival rate of the NSCLC patients.
Periostin was first discovered as a new type of bone adhesion molecule by subtractive hybridisation and differential screening in the cDNA library of murine osteoblast cell line, which is a unique extracellular matrix protein secreted by the osteoblasts and its precursors.11,12 Periostin can regulate cell adhesion, diffusion, and growth by binding to the integrins αγδ1, 3, 5, aggregating epithelial cell growth receptors, and activating FAK and Akt/PKB-mediated signal arches transduction.13,14 Tumour cells usually grow and proliferate rapidly in the harsh environments such as ischemia and hypoxia, and even further metastasise while resisting various anti-tumour factors produced by the body.15 A large number of studies have shown that under severe conditions, periostin can promote tumour proliferation in head and neck cancer.16 Angiogenesis is the basic process in the occurrence and development of malignant tumours, which is closely related to the metastasis and prognosis of various malignant tumours . However, relevant research shows that periostin plays an important role in the process of promoting angiogenesis.17 Therefore, this study aimed to explore whether periostin could be used as a reliable marker for the early clinical diagnosis and prognosis of NSCLC.
METHODOLOGY
A total of 66 NSCLC patients, who were treated in General Hospital of Ningxia Medical University, from October 2018 to October 2019, were consecutively adopted as the NSCLC group. Another 40 patients with benign lung lesions, and 38 healthy volunteers, examined during the same period, were selected and named as the benign group and control group, respectively. The NSCLC group inclusion criteria were: diagnosis of NSCLC according to the “standardised diagnosis and staging treatment on non-small cell lung cancer;”18 underwent radical resection of lung cancer; aged 18~75 years; not received relevant radiotherapy and chemotherapy in the past one month; and had given informed and voluntarily consent for the study. The exclusion criteria were presence of cerebral haemorrhage or any other malignancy; refusal to participate in the study; severe dysfunction of heart, liver, and kidney; and physically unfit for surgery.
Cubital vein blood (5 mL) was collected from all the subjects the next morning after admission. After centrifugation at 2500 r/min for ten minutes, the supernatant was collected and stored at -20°C for future use. The serum levels of periostin in all the patients were determined by enzyme-linked immunosorbent assay (ELISA). Periostin kit was purchased from Shanghai Jingkang Biological Engineering Co., Ltd., and the experimental procedures were carried out in strict accordance with the kit instructions. After one year of follow-up, the above method was used to detect the serum periostin levels of the patients in the NSCLC group. The serum periostin levels of NSCLC patients with recurrence and metastasis, and those without recurrence and metastasis were compared.
The research data were imported into SPSS 22.0 statistical software for processing and analysis. The measurement data were expressed using mean±standard deviation, and the comparison was performed by Student’s t-test. The categorical variables were expressed by n (%). The prognostic effect of the periostin level was evaluated by the receiver operating characteristic (ROC) curve. A difference was considered significant when the p-value was less than 0.05.
RESULTS
NSCLC group included 46 (69.7%) males and 20 (30.3%) females with an average age of 53.43±8.42 years. Tumour location was upper lobe in 26 (39.4%), middle lobe in 21 (31.8%), and lower lobe in 19 (28.8%) cases. Tumour type was adenocarcinoma in 27 (40.9%), squamous cell carcinoma in 20 (30.3%) cases, and 19 (28.8%) had other histology. The benign group included 28 (70.0%) males and 12 (30.0%) females with an average age of 52.05±8.32 years. Twenty six (68.4%) males and 12 (31.6%) females were included in the control group, and the average age was 52.50±7.90 years.
Comparison of serum periostin levels in NSCLC patients, benign lung diseases, and healthy controls: the serum periostin level in patients with NSCLC was obviously higher than that of benign patients with lung disease and healthy controls (p<0.001); there was not apparently statistical difference between the benign lung disease group and the healthy control group (p=0.201, Table I).
There was a statistical difference in serum periostin levels among NSCLC patients with different T stages. Serum periostin levels in patients at T3 stage were higher than that of T2 and T1 stages (p=0.014 and p<0.001, respectively), and T2 stage was higher than T1 stage (p<0.001), as seen in Table I.
There was a statistical difference in serum periostin levels among NSCLC patients with different N stages, the serum Periostin levels in patients at N2 stage were higher than that of N1 and N0 stages (p=0.003 and p<0.001, respectively), and N1 stage was higher than N0 stage (p<0.001, Table I).
Compared with the postoperative non-recurrent NSCLC patients, the postoperative recurrent NSCLC patients had higher serum periostin level, the difference has statistical significance (p<0.001, Table I). The serum level of NSCLC patients with tumour metastasis was significantly higher than that of the patients without metastasis (p<0.001), as shown in Table I.
The evaluation value of serum periostin level in the prognosis of NSCLC patients: the ROC curve showed that the AUC of serum periostin level evaluating postoperative recurrence of NSCLC patients was 0.739, the sensitivity was 69.23%, and the specificity was 75.47% at the value of 54.12 ng/mL, as seen in Figure 1. The AUC of serum Periostin level evaluating postoperative metastasis of NSCLC patients was 0.831, the sensitivity was 80.00%, and the specificity was 82.93% at the value of 42.84 ng/mL (Figure 2).
DISCUSSION
NSCLC is a major type of lung cancer characterised by the slow cancer cell division and proliferation, as well as later metastasis. However, when the first clinically diagnosed, the NSCLC patients have developed into the middle-advanced stage, resulting in poor prognosis, and the 5-year survival rate is only about 20%. In the decade years, with the continuous improvement of the medical level and the in-depth research of the pathobiology of lung cancer, the surgical treatment of NSCLC was relatively mature and has made great progress, but the choice of surgical methods is still controversial.
Table I: The serum periostin levels among different groups and clinical features.|
Groups / features |
n |
Percentages (%) |
Periostin (ng/mL) |
p |
|
NSCLC group/ Benign group |
66/40 |
- |
37.94±9.13/27.85±5.84 |
<0.001 |
|
Benign group/ Control group |
40/38 |
- |
27.85±5.84/26.38±4.02 |
0.201 |
|
NSCLC group/Control group |
66/38 |
- |
37.94±9.13/26.38±4.02 |
<0.001 |
|
T stages |
|
|
|
|
|
T3/ T2 |
16/24 |
- |
52.01±10.79/44.28±8.11 |
0.014 |
|
T2/ T1 |
24/26 |
- |
44.28±8.11/33.63±9.52 |
<0.001 |
|
T3/T1 |
16/26 |
- |
52.01±10.79/33.63±9.52 |
<0.001 |
|
N stages |
|
|
|
|
|
N2/ N1 |
17/26 |
- |
51.08±12.49/38.95±12.07 |
0.003 |
|
N1/ N0 |
26/23 |
- |
38.95±12.07/29.08±7.20 |
0.001 |
|
N2/N0 |
17/23 |
- |
51.08±12.49/29.08±7.20 |
<0.001 |
|
Postoperative recurrence |
|
|
|
|
|
Yes |
13 |
19.7 |
60.60±15.56 |
<0.001 |
|
No |
53 |
80.3 |
47.47±10.20 |
|
|
Tumour metastasis |
|
|
|
|
|
With |
25 |
37.9 |
49.13±12.79 |
<0.001 |
|
Without |
41 |
62.1 |
35.18±8.20 |
|
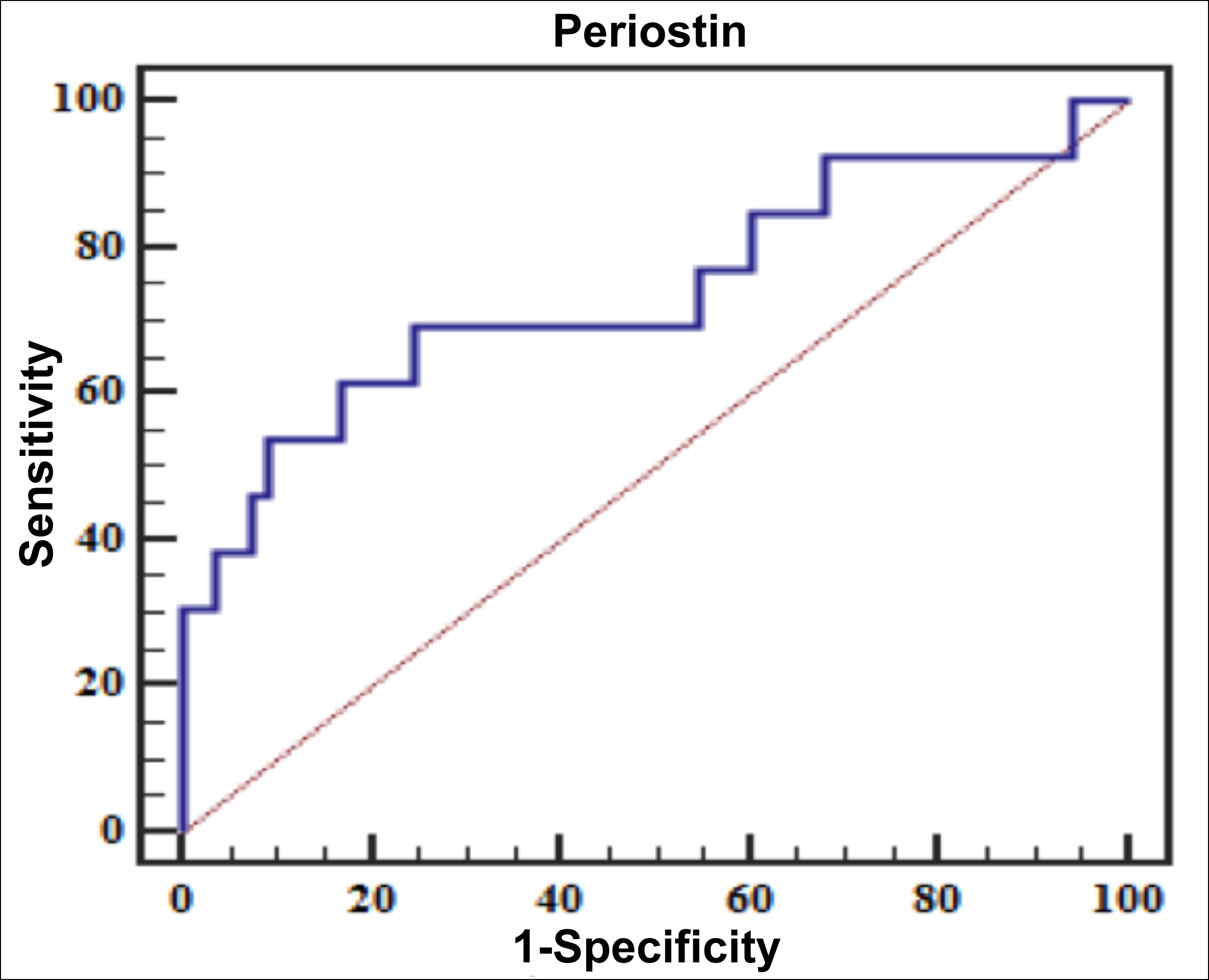 Figure 1: ROC curve of serum periostin level in NSCLC patients to predict postoperative recurrence.
Figure 1: ROC curve of serum periostin level in NSCLC patients to predict postoperative recurrence.
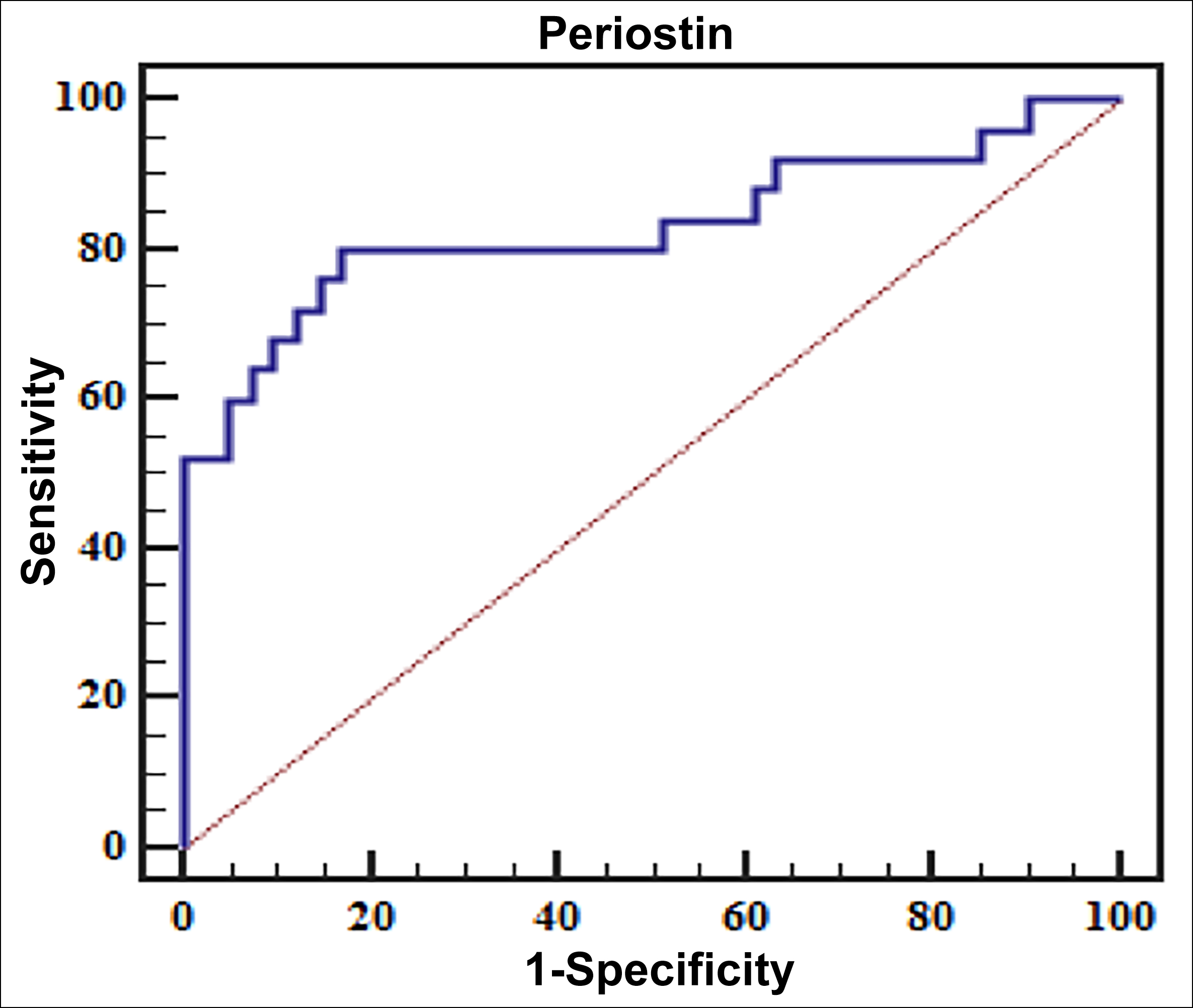 Figure 2: ROC curve of serum periostin level predicting postoperative metastasis in the NSCLC patients.
Figure 2: ROC curve of serum periostin level predicting postoperative metastasis in the NSCLC patients.
Therefore, it is urgent to identify effective biological factors to achieve accurate preoperative staging and prognostic evaluation of NSCLC patients. Periostin, known as osteoblast-specific factor 2 (OSF-2), is a multi-functional extracellular matrix protein secreted by the osteoblasts.19 It participates in the pathological and physiological development of various malignant tumours by regulating cell adhesion, osteoblast proliferation, and differentiation. In addition, it has been reported that periostin is highly expressed in different types of the tumours such as pancreatic cancer,20 ovarian cancer, and prostate cancer.21,22 Therefore, in recent years, periostin has received extensive attention from the medical researchers. Underwood's research team found that the periosteal protein can predict the prognosis of tube cancer and promote the erosion of esophageal cancer.23 Erkan et al. discovered that the mesenchymal cells in the primary tumour and metastases of pancreatic ductal adenocarcinoma (PDAC) produce periostin.24 Through in vitro tumour strain mechanics microenvironment and tumour hypoxic microenvironment simulation experiments, it is found that the periosteum protein may be a positive regulator of tumour growth, and promotes the survival of the cancer cells by activating the Akt/PKB pathway.25
Therefore, this study focused on exploring the expression of periostin in NSCLC patients and its significance in clinical staging and prognostic evaluation.
ROC was used to determine the value of periostin level for predicting postoperative recurrence and metastasis. In ROC curve, the AUC predicting NSCLC postoperative recurrence was 0.739, with 69.23% sensitivity and 75.47% specificity at the 54.12 ng/mL periostin level; the AUC predicting NSCLC postoperative metastasis was 0.831, with 80.00% sensitivity and 82.93% specificity at the 42.84 ng/mL Periostin level, indicating the serum Periostin level has a certain evaluation value for the prognosis of NSCLC patients; however, the value for predicting metastasis was greater than predicting recurrence. In short, Serum periostin level is closely linked to the occurrence and development of NSCLC which can preliminarily reflect the tumour stage and the degree of deterioration of the patient, and has shown certain evaluation value for the prognosis of the NSCLC patients. This research is beneficial to provide theoretical basis for clinical staging, diagnosis, and prognosis evaluation for NSCLC patients and of vital importance for improving the survival rate of the patients. However, there are still some shortcomings in this study, such as the number of samples included is limited and the follow-up time is too short. In the next stage, it is necessary to expand the sample size and follow-up time for further research.
CONCLUSION
Serum periostin level is possibly related to the progression of NSCLC, and exhibited certain predictive value for the prognosis of NSCLC patients, and the value of periostin level predicting metastasis was greater than predicting recurrence.
ETHICAL APPROVAL:
This study was approved by the Local Ethics Committee and performed in accordance with the Declaration of Helsinki.
PATIENTS’ CONSENT:
Informed consents were obtained from the patients.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
ZQ, LZ, JH, WH: Drafted the work and revised it critically for important intellectual content.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Shapiro M, Mhango G, Kates M, Weiser TS, Chin C, Swanson SJ, et al. Extent of lymph node resection does not increase perioperative morbidity and mortality after surgery for stage I lung cancer in the elderly. Eur J Surg Oncol 2012; 38(6):516-22. doi: 10.1016/j.ejso.2011.12.018.
- Thomas PA, Berbis J, Baste JM, Pimpec-Barthes FL, Tronc F, Falcoz PE, et al. Bilobectomy for lung cancer: Contemporary national early morbidity and mortality outcomes. J Thorac Cardiovasc Surg 2015; 149(1):73-83. doi: 10.1016/j. jtcvs.2014.09.063.
- Su Z, Dias-Santagata D, Duke M, Hutchinson K, Lin YL, Borger DR, et al. A platform for rapid detection of multiple oncogenic mutations with relevance to targeted therapy in non-small-cell lung cancer. J Molecular Diagn 2018; 13(1): 74-84. doi: 10.1016/j.jmoldx.2010.11.010.
- Lee HY, Choi WH, Yoo IR, Park JK, Sung SW, Kim YS, et al. Prognostic value of 18F‐FDG PET parameters in patients with locally advanced non‐small cell lung cancer treated with induction chemotherapy. Asia Pac J Clin Oncol 2020; 16(1):70-4. doi: 10.1111/ajco.13288.
- Nakayama Y, Nonaka T, Mizoguchi N. Prognostic value of histopathological response to preoperative therapy for the patients with non-small cell lung cancer. Int J Radiation Oncol Biol Physics 2012; 84(3):607.
- de Boer RH, Arrieta O, Yang CH, Gottfried M, Chan V, Raats J, et al. Vandetanib plus pemetrexed for the second-line treatment of advanced non-small-cell lung cancer: A randomised, double-blind phase III trial. J Clin Oncol 2011; 29(8):1067-74. doi: 10.1200/JCO.2010.29.5717.
- Osarogiagbon RU, Lee YS, Faris NR, Ray MA, Ojeabulu PO, Smeltzer MP. Invasive mediastinal staging for resected non-small cell lung cancer in a population-based cohort. J Thorac Cardiovas Surg 2019; 158(4):1220-9. doi: 10. 1016/j.jtcvs. 2019.04.068.
- Ohnishi R, Yasuda I, Kato T, Kaneko Y, Suzuki T, Yasuda S, et al. Combined endobronchial and endoscopic ultrasound-guide fine needle aspiration for mediastinal nodal staging of lung cancer. Endoscopy 2011; 43(12):1082-9. doi: 10.1055/s-0030-1256766.
- Dincer HE, Gliksberg EP, Andrade RS. Endoscopic ultrasound and / or endobronchial ultrasound-guided needle biopsy of central intraparenchymal lung lesions not adjacent to airways or esophagus. Endoscopic Ultrasound 2015; 4(1):40-3. doi: 10.4103/2303-9027.151332.
- Rice SR, Molitoris JK, Vyfhuis M. Magnetic resonance imaging brain staging in stage I-III non-small cell lung cancer (NSCLC): Incidence and clinicopathologic factors in asymptomatic brain metastases at diagnosis. Int J Radiation Oncol Biol Physic 2016; 96(2):480-1.
- Kyutoku M, Taniyama Y, Katsuragi N, Shimizu H, Kunugiza Y, Iekushi K, et al. Role of periostin in cancer progression and metastasis: Inhibition of breast cancer progression and metastasis by anti-periostin antibody in a murine model. Int J Molecular Med 2011; 28(2):181-6. doi: 10.3892/ijmm. 2011.712.
- Field S, Uyttenhove C, Stroobant V, Cheou P, Donckers D, Coutelier JP, et al. Novel highly specific anti-periostin antibodies uncover the functional importance of the fascilin 1-1 domain and highlight preferential expression of periostin in aggressive breast cancer. Int J Cancer 2016; 138(8): 1959-70. doi: 10.1002/ijc.29946.
- Hu Q, Tong S, Zhao X, Ding W, Gou Y, Xu K, et al. Periostin mediates TGF-β-induced epithelial mesenchymal transition in prostate cancer cells. Cell Physiol Biochem Pharmacol 2015; 36(2):799-809. doi: 10.1159/000430139.
- Orecchia P, Conte R, Balza E, Castellani P, Borsi L, Zardi L, et al. Identification of a novel cell binding site of periostin involved in tumour growth. Eur J Cancer 2011; 47(14): 2221-9. doi: 10.1016/j.ejca.2011.04.026.
- Ben-Ari Z, Hochhauser E, Papo O, Kaganovsky E, Krasnov T, Vamichkim A, et al. Role of anti-tumour necrosis factor-alpha in ischemia/reperfusion injury in isolated rat liver in a blood-free environment. Transplantation 2002; 73(12):1875-80. doi: 10.1097/00007890-200206270- 00004.
- Liu C, Feng X, Wang B, Wang X, Wang C, Yu M, et al. Bone marrow mesenchymal stem cells promote head and neck cancer progression through periostin mediated PI3K/AKT/mTOR. Cancer Sci 2017; 109(3):688-98. doi: 10.1111/cas.13479.
- Shao R, Bao S, Bai X, Blanchette C, Anderson RM, Dang T, et al. Acquired expression of periostin by human breast cancers promotes tumour angiogenesis through up-regulation of vascular endothelial growth factor receptor 2 expression. Mol Cell Biol 2004; 24(9):3992-4003. doi: 10.1128/MCB.24.9.3992-4003.2004.
- Huang C. Standardised diagnosis and staging treatment on non-small cell lung cancer. Caribbean J Ofence 2005; 41(3):465-75.
- Liu Y, Du L. Role of pancreatic stellate cells and periostin in pancreatic cancer progression. Tumour Biol 2015; 36(5): 3171-7. doi: 10.1007/s13277-015-3386-2.
- Choi KU, Yun JS, Lee IH, Chul Heo S, Shin SH, Jeon ES, et al. Lysophosphatidic acid-induced expression of periostin in stromal cells: Prognoistic relevance of periostin expression in epithelial ovarian cancer. Int J Cancer 2011; 128(2): 332-42. doi: 10.1002/ijc.25341.
- Lee YJ, Kim IS, Park SA, Kim Y, Lee JE, Noh DY, et al. Periostin-binding DNA aptamer inhibits breast cancer growth and metastasis. Mol Ther 2013, 21(5):1004-13. doi: 10.1038/mt. 2013.30.
- Yuan T, Choi CH, Li QK, Rahmatpanah FB, Chen X, Kim SR, et al. Overexpression of periostin in stroma positively associated with aggressive prostate cancer. PLOS One 2015; 10(3):1-10. doi: 10.1371/journal.pone.0121502.
- Underwood TJ, Hayden AL, Derouet M, Garcia E, Noble F, White MJ, et al. Cancer-associated fibroblasts predict poor outcome and promote periostin-dependent invasion in oesophageal adenocarcinoma. J Pathol 2015; 235(3): 466-77. doi: 10.1002/path.4467.
- Erkan M, Klee FfJ, Gorbachevski A, Reiser C, Mitkus T, Esposito I, et al. Periostin creates a tumour-supportive microenvironment in the pancreas by sustaining fibrogenic stellate cell activity. Gastroenterol 2007; 132(4):1447-64. doi: 10.1053/j.gastro.2007.01.031.
- Ouyang G, Liu M, Ruan K, Song G, Mao Y, Bao S, et al. Upregulated expression of periostin by hypoxia in non-small-cell lung cancer cells promotes cell survival via the Akt/PKB pathway. Cancer Lett 2009; 281(2):213-9. doi: 10.1016/j.canlet.2009.02.030.