CeO2 Nanoparticles Effect on Acute Hemorrhagic Shock Induced-hepatic Stress Injury in a Mouse Model
By Guoqiang ChenAffiliations
doi: 10.29271/jcpsp.2022.06.728ABSTRACT
Objective: To explore the effects of CeO2 nanoparticles on liver injury, inflammation, oxidative stress, and coagulation function in hemorrhagic shock (HS)-induced hepatic stress injury.
Study Design: Experimental study.
Study Place and Duration: Shanghai Tenth People's Hospital, Tongji University, School of Medicine, Shanghai, China, from March 2017 to July 2020.
Methodology: HS mice were treated with different doses of CeO2 nanoparticles suspension (1 mg/ml), 0.1 ml/100 g, 0.2 ml/100 g, 0.5 ml/100 g. Levels of NF-κB, IFN-γ, IL-1β, IL-6, and TNF-α in tissue homogenate were measured by ELISA. Oxidative stress-related factors in liver tissue homogenate were also evaluated. Coagulation function was determined by measurement of the prothrombin time (PT) and activated partial thromboplastin time (APTT) as well as using thromboelastography analysis. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were detected for liver function. Survival analysis within 24 hours after the model establishment was conducted by K-M curve.
Results: Hemorrhagic shock resulted in obvious tissue hemorrhage, elevated levels of AST and ALT, and significantly shorter survival for HS mice, which was improved by 0.5 ml/100 g CeO2 nanoparticles suspension. Treatment of CeO2 nanoparticles significantly decreased HS-induced inflammation, oxidative stress, and coagulation function in a dose-dependent manner.
Conclusion: CeO2 nanoparticles could suppress inflammation and oxidative stress, as well as improve liver and coagulation function in hemorrhagic shock-induced hepatic stress injury mice.
Key Words: CeO2 nanoparticles, Hemorrhagic shock-induced hepatic stress injury, Inflammatory response, Oxidative stress, Coagulation function.
INTRODUCTION
Hemorrhagic shock (HS) can cause a series of organ stress dysfunction and injury, including hepatic injury, lung injury, and gastrointestinal tract dysfunction.1,2 Generally, it is considered that the dysfunction of the hypothalamus, immune and endocrine function, as well as the release of inflammatory factors and oxidative stress, are associated with the hepatic stress injury, which may finally lead to multiple organ dysfunction syndromes (MODS).3
In biological contexts, it has been reported that Cerium oxide (CeO2) nanoparticles can mimic enzymatic antioxidants such as superoxide dismutase and catalase.4,5 Cerium atoms have two oxidation states (Ce3+ and Ce4+) in this structure which they could easily convert to each other due to the small amount of energy difference for comparative occupancy of 4f and 5d orbitals. Also, there is some oxygen vacancies in the ceria lattice structure which occur during redox reactions. These properties employed it as a biological antioxidant agent. NC has been applied for biological purposes due to its low toxicity and worthy antioxidant properties. The beneficial effects of CeO2 nanoparticles have been reported in different clinical conditions associated with the reduction of ROS such as stroke, diabetes, inflammation, and cancer.6-9 The authors’ previous study also confirmed that cerium oxide nanoparticles can reduce the release of inflammatory and proinflammatory factors in sepsis and reduce the damage to the liver.10 However, the effects of CeO2 nanoparticles on hepatic stress injury after a hemorrhagic shock are still unknown.
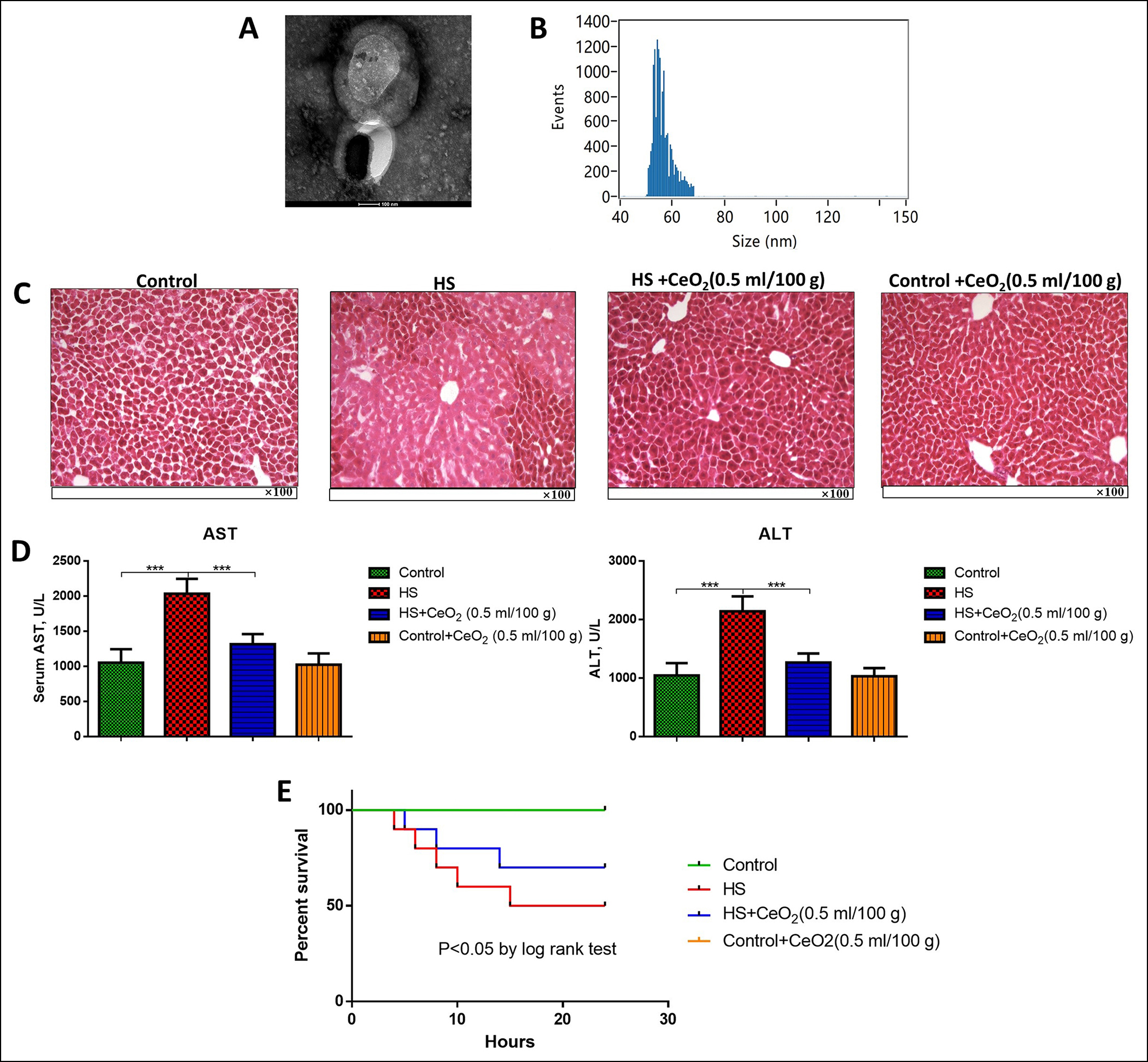 Figure 1: (A) Morphology of CeO2 nanoparticles. (B) Diameter of CeO2 nanoparticles. (C) HE staining for liver tissues of different groups of mice (n=6 in each group). (e) Serum ALT and AST levels of different groups of mice (n=6 in each group). (D) K-M curve for survival analysis of different groups of mice (n=10 in each group). ***p<0.001.
Figure 1: (A) Morphology of CeO2 nanoparticles. (B) Diameter of CeO2 nanoparticles. (C) HE staining for liver tissues of different groups of mice (n=6 in each group). (e) Serum ALT and AST levels of different groups of mice (n=6 in each group). (D) K-M curve for survival analysis of different groups of mice (n=10 in each group). ***p<0.001.
This study might provide novel research targets for the treatment of hepatic stress injury after hemorrhagic shock. The objective of this study was to explore the effects of CeO2 nanoparticles on liver injury, inflammation, oxidative stress, and coagulation function in hemorrhagic shock (HS)-induced hepatic stress injury.
METHODOLOGY
It was an experimental animal-model study conducted at Shanghai Tenth People's Hospital, Tongji University, School of Medicine, Shanghai, China from March 2017 to July 2020 after approval from the Animal Care Ethics Commission of the Hospital.
CeO2 nanoparticles (M.W. 172.11, diameter <100 nm) were purchased from Aladdin (cat. No. C103984, Shanghai, China). It was synthesized by a precipitation method. In brief, 1.736 g Ce(NO3)3 was added to NaOH solution (0.4 g NaOH dissolved in 128 mL water) followed by 48 h of magnetic stirring. The resulting white precipitate was collected and washed several times in ultrapure water. The suspension of CeO2 was prepared by dissolving CeO2 nanoparticles into PBS to 1 mg/ml. The solution was then shaken in an ultrasonic cell breaker (VCX500, Sonics, USA) for 2 min and filtered successively through a 0.20 µm-diameter membrane. For identification of CeO2 nanoparticles, the nanoparticles were observed under a scanning electron microscope (S-4800 Japanese HITACHI Company), transmission electron microscopy (TEM, JEM-2010, JEOL, Tokyo, Japan), Ultraviolet-visible spectroscopy (Thermo Scientific Evolution 300 UV–Vis Spectrophotometer, Germany), Fourier transform infrared spectroscopy (FTIR, ST-IRST-SIR spectrometer), and X-ray diffraction (XRD, X’pert PRO MPD, PANalytical, Almelo, The Netherlands). The average particle size of CeO2 nanoparticles was measured by high sensitivity flow cytometry for nanoparticle analysis using an FSCAN flow cytometer (BD Biosciences, USA). The stability of CeO2 nanoparticles was determined with the ITLC method.
Briefly, 60 male BALB/C mice (8~12 weeks, weighed 25~30 g) were purchased from SJA Laboratory Animal company (Hunan, China). The mice all got free access to food and water in micro-isolator cages. This study was approved by the Animal Ethics Committee of Nantong University, China.
For the establishment of the hemorrhagic shock model, bilateral inguinal dissections were performed, followed with a small femoral arteriotomy. The hemorrhagic shock model was established by taking stepwise blood via the carotid artery until the mean arterial pressure reached 25±2 mmHg for 120 minutes. The hemodynamics was continuously monitored using a contralateral cannula. Then, 3 times the volume of shed blood with Ringer's lactate were transfused to the mice for the resuscitation, with the body temperature maintained at 37°C. At 2 hours after hemorrhagic shock and resuscitation (HS-R), mice were euthanised. The sham group received all procedures without HS-R.
For treatment of CeO2 nanoparticles, mice received tail vein injection of different doses of CeO2 nanoparticles suspension (1 mg/ml) before the surgery: low dose 0.1 ml/100 g (bodyweight), middle dose 0.2 ml/100 g, high dose 0.5 ml/100 g. The blank control received the same volume of normal saline. Each group had 6 mice in every experiment.
After 2 h of model establishment, liver tissues of the mice were extracted and HE staining was performed to evaluate the necrosis and inflammatory infiltrate after fixation, dehydration, and xylene infiltration.
After 2 h of model establishment, animals were sacrificed and liver tissues were collected and pounded to pieces. After centrifugation at ×12000 g under room temperature for 15 min, the supernatants were collected. Then, levels in tissue homogenate of inflammation-related factors of NF-κB, IFN-γ, IL-1β, IL-6, and TNF-α were measured by ELISA using corresponding ELISA kits (all purchased from Abcam, Cambridge, MA, USA).
The levels of superoxide dismutase (SOD), malondialdehyde (MDA), and reactive oxygen species (ROS) in liver tissue homogenate were measured after 2 h of model establishment using commercial kits from Nanjing Jiancheng Bio-Technology Co., Ltd. according to manufacturer’s instruction. After 2 h of model establishment, platelet function and coagulopathy were measured by thromboelastography analysis using Haemoscope 5000 analyzers (Haemonomics, Braintree, MA). The boardastography software (version 4.2.3, Haemonomics) was used for the generation of coagulation profiles. Maximal amplitude (MA, in millimeters) was reported as a measure of cluster strength and plan aggregation/function. Serum levels of ALT and AST were measured after 2 h of model establishment using the Dri-Chem 7000 Chemistry Analyzer (Heska Co, Loveland, CO; slides from Fujifilm Japan) according to the manufacturer’s instructions. Briefly, after 2 h of model establishment, blood samples were collected in an anticoagulant tube. After centrifugation ×10000 g under room temperature for 15 min, the plasma was obtained and the prothrombin time (PT) and activated partial thromboplastin time (APTT) were evaluated using an Automatic Coagulation Analyzer (ACL-TOP700, Beckman, USA).
The measurement data were expressed by mean ± SD. Comparisons were conducted using a one-way analysis of variance (ANOVA) followed by a Tukey post hoc test for three or more groups. Student t-test was used for comparison between two groups. Survival analysis within 24 h after the model establishment was conducted by K-M curve. P <0.05 was considered as statistically different. All calculations were made using Graphpad 6.0.
RESULTS
The CeO2 nanoparticles were observed under a scanning electron microscope and the average particle size was measured (Figure 1A-B). The effects of CeO2 nanoparticles on hepatic stress injury were then determined. Mice were treated with a high dose of CeO2 nanoparticles (0.5 ml/100 g). The model group showed obvious tissue hemorrhage, which was improved by CeO2 treatment (Figure 1C). The levels of AST and ALT were both elevated in hemorrhage mice compared with the sham group (p<0.05). However, when treated with CeO2 nanoparticles, serum levels of ALT and AST significantly decreased in a dose-dependent manner (p<0.05, Figure 1D). K-M curve showed mice in the HS group had significantly shorter survival while treatment of CeO2 nanoparticles markedly prolonged the survival time (p<0.05, Figure 1E). Besides, in all experiments, the group of control-treated with CeO2 nanoparticles showed no significant difference from the control group, indicating the CeO2 nanoparticles did not influence normal animals.
To further investigate the effects of CeO2 nanoparticles on acute hemorrhagic shock-induced hepatic stress injury, mice were treated with different doses of CeO2 nanoparticles. It was observed that inflammatory factors of NF-κB, IFN-γ, IL-1β, IL-6, and TNF-α were all markedly increased in hemorrhage mice in liver tissues (p<0.05, Figure 2A).
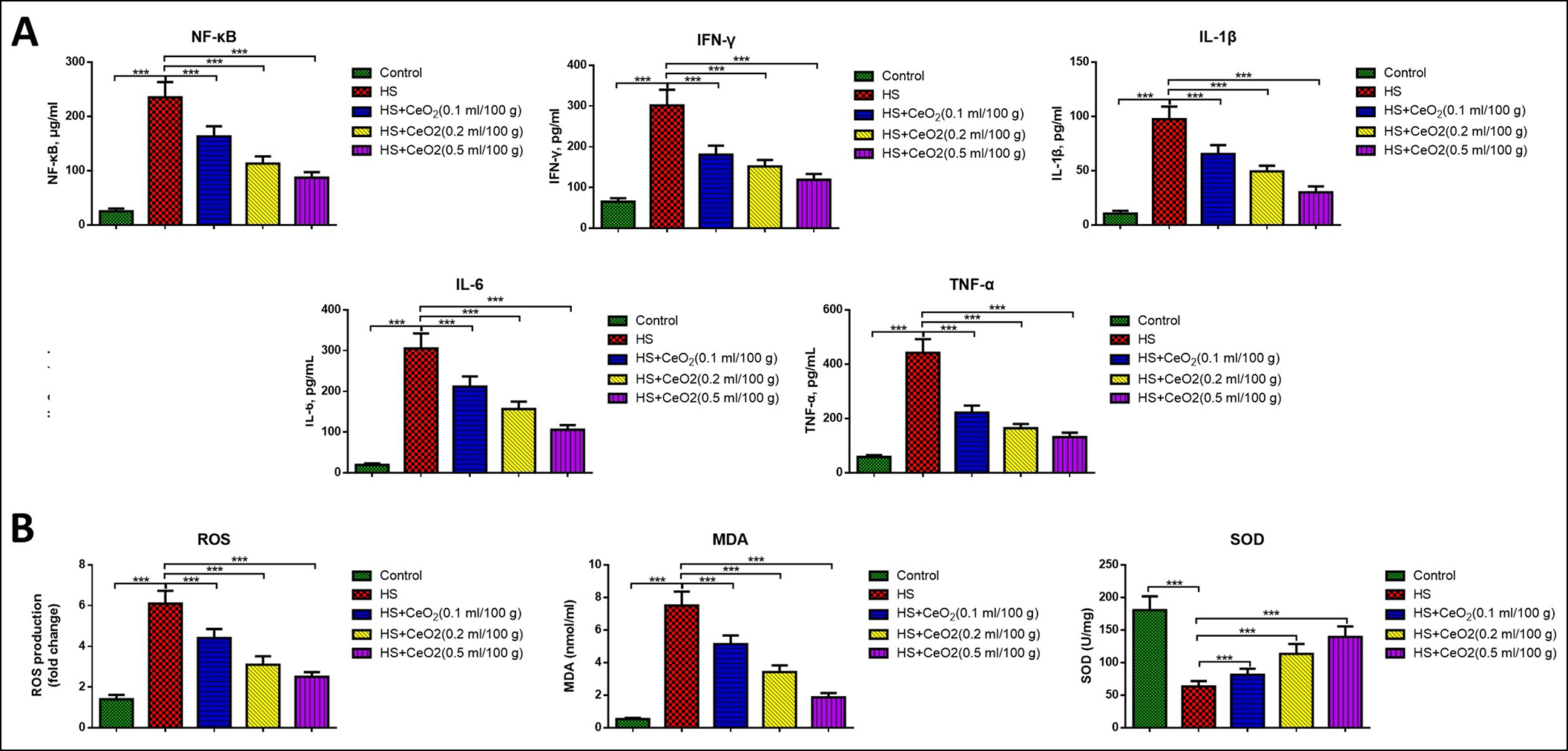 Figure 2: (A) Inflammatory factors of NF-κB, IFN-γ, IL-1β, IL-6 and TNF-α in liver tissues of different groups of mice (n=6 in each group). (B) Oxidative stress factors ROS, SOD and MDA in liver tissues of different groups of mice (n=6 in each group). ***p<0.001.
Figure 2: (A) Inflammatory factors of NF-κB, IFN-γ, IL-1β, IL-6 and TNF-α in liver tissues of different groups of mice (n=6 in each group). (B) Oxidative stress factors ROS, SOD and MDA in liver tissues of different groups of mice (n=6 in each group). ***p<0.001.
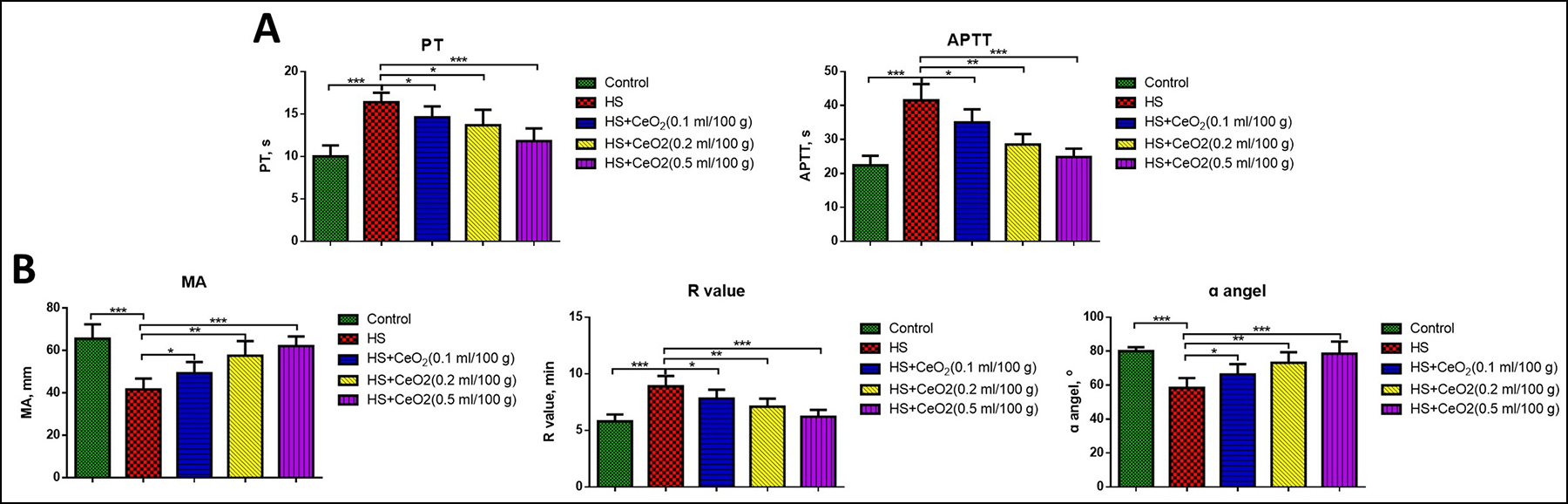 Figure 3: (A) Levels of PT and APTT in different groups of mice (n=6 in each group). (B) R value, MA and ɑ angel in different groups of mice by thromboelastography analysis (n=6 in each group). *p<0.05, p<0.01, ***p<0.001.
Figure 3: (A) Levels of PT and APTT in different groups of mice (n=6 in each group). (B) R value, MA and ɑ angel in different groups of mice by thromboelastography analysis (n=6 in each group). *p<0.05, p<0.01, ***p<0.001.
When treated with CeO2 nanoparticles, the inflammatory factors were all markedly reduced in a dose-dependent manner (P<0.05). Similarly, the oxidative stress factors ROS and MDA significantly increased and SOD remarkably decreased in hemorrhage mice in liver tissues (p<0.05, Figure 2B). While treatment with CeO2 nanoparticles remarkably reduced the levels of ROS and MDA and increased the levels of SOD in liver tissues compared with the model mice, and the effects were also in a dose-dependent manner (p<0.05), suggesting that the CeO2 nanoparticles reduced both inflammation and oxidative stress in hemorrhagic shock-induced hepatic stress injury.
At last, we investigated the role of CeO2 nanoparticles in coagulation function in hemorrhagic shock mice. As shown in Figure 3A, both levels of PT and APTT remarkably increased in hemorrhage mice compared with the sham group, which was significantly decreased by treatment of CeO2 nanoparticles in a dose-dependent manner (p<0.05). Then, thromboelastography analysis was used to further evaluate the coagulation function. It was found the R-value significantly increased, while the values of MA and ɑ angel significantly decreased in hemorrhage mice compared with the sham group (Figure 3B). The treatment of CeO2 nanoparticles markedly decreased the R value and increased the values of MA and ɑ angel in a dose-dependent manner (p<0.05).
DISCUSSION
Despite numerous researches, treatment of hemorrhagic shock-induced hepatic stress injury is still a clinical challenge.2,11 In recent years, the effects of CeO2 nanoparticles have been noticed in many diseases.12 However, whether CeO2 nanoparticles can improve hemorrhagic shock-induced hepatic stress injury is not clear. In this study, it was observed that treatment of CeO2 nanoparticles could improve liver injury, suppress inflammation and oxidative stress, as well as improve liver and coagulation function in hemorrhagic shock-induced hepatic stress injury mice.
Hemorrhagic shock could induce a series of organ dysfunctions, including hepatic stress injury and studies also reported potential methods to treat it. Wagner et al. demonstrated that ethyl pyruvate improved alanine aminotransferase levels and liver injury by inhibition of local inflammation, NF-κB activation, and HMGB1 release.13 Another study showed that Carboxyfullerene nanoparticles could improve acute hepatic injury by suppressing NF-κB and inflammatory response in severe hemorrhagic shock.14 In a recent research, Liu et al. found Corilagin protected the liver after hemorrhagic shock by decreasing CINC-1 and CINC-3 through activating Akt signaling.15 During hepatic stress injury, both inflammation, and oxidative stress will be activated. It was found in hemorrhagic shock, the levels of MDA and MPO, and inflammatory factor TNF-α were elevated in the liver, intestine, lungs, and brain.16 Besides, the production of superoxide anion and reactive oxidants, as well as neutrophil degranulation were also elevated after hemorrhagic shock.17 In this research, we also found that hemorrhagic shock could induce liver injury, with increased inflammation and activated oxidative stress, as well as liver dysfunction.
Except for inflammation and activated oxidative stress, the coagulation function is also considered to be damaged during liver injury. After the hemorrhagic shock, the international normalized ratio, thrombin time, and prothrombin time, as well as D-dimer were all observed to be increased while the platelet count and fibrinogen concentration were decreased.18 Ding et al. showed coagulation dysfunction and organ injury after hemorrhagic shock and resuscitation were associated with toll-like receptor 4 (TLR4), which contributed to coagulopathy.19 In this research, coagulation dysfunction with increased PT and APTT, increased R-value and decreased MA value and ɑ angel in thromboelastography analysis were observed.
CeO2 nanoparticles have many bioactivities, including anti-inflammation and anti-oxidation. In an early study, it was found CeO2 nanoparticles could reduce the levels of total nitrated proteins, MCP-1 and CRP in MCP-1 transgenic mice.20 In another study, Gojova et al. found that CeO2 induced a slight inflammatory response in human aortic endothelial cells.21 In a recent research, CeO2 nanoparticles were reported to have the potential as a treatment method for inflammatory bowel disease, in which an orally administered CeO2 nanozyme could reduce inflammation through ROS scavenging.22 Besides, in psoriasis, CeO2 nanoparticles were found to activate superoxide dismutase- and catalase-mimicking activities, and protect against ROS-mediated damage.23 Tsai et al. also demonstrated that CeO2 nanoparticles could improve airway mucus secretion, and protect cells by diminishing ROS and inflammatory responses induced by TiO2 nanoparticles.24 Moreover, CeO2 nanoparticles were wildly used in orthopedic biomedicine, in particular, bone tissue engineering (BTE).25 In this study, the author demonstrated that CeO2 nanoparticles could improve hemorrhagic shock-induced hepatic stress injury through suppressing inflammation, oxidative stress and improving liver and coagulation function in a dose-dependent manner.
CONCLUSION
In this in vivo study, it was found that CeO2 nanoparticles could improve hemorrhagic shock-induced hepatic stress injury through inhibition of inflammation, oxidative stress, and improvement of liver and coagulation function. This study could provide a research basis and potential therapeutic methods for the application of CeO2 nanoparticles in the treatment of hemorrhage-induced hepatic stress injury.
ETHICAL APPROVAL:
This study was conducted after approval from the Animal Care Ethics Commission of the Shanghai Tenth People's Hospital, Tongji University, School of Medicine, Shanghai.
PATIENTS' CONSENT:
All participants had signed the informed consent.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHOR’S CONTRIBUTION:
GC: Substantial contribution to the conception and design of the work; and the acquisition, analysis, and interpretation of data for the work; drafting the work and revising it critically for important intellectual content; final approval of the version to be published; agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy and integrity of any part of the work are appropriately investigated and resolved.
REFERENCES
- Luís A, Hackl M, Jafarmadar M, Keibl C, Jilge JM, Grillari J, et al. Circulating miRNAs associated with ER Stress and organ damage in a preclinical model of trauma hemorrhagic shock. Frontiers Med 2020; 7:588. doi: 10.3389/fmed. 2020.568096.
- Chang R, Holcomb JB. Optimal fluid therapy for traumatic hemorrhagic shock. Critical Care Clinics. 2017; 33(1):15-36. doi: 10.1016/j.ccc.2016.08.007.
- Pfeifer R, Lichte P, Schreiber H, Sellei RM, Dienstknecht T, Sadeghi C, et al. Models of hemorrhagic shock: Differences in the physiological and inflammatory response. Cytokine 2013; 61(2):585-90. doi: 10.1016/j.cyto.2012.10.022.
- Si S, Li L, Wang Z, Wu Y, Shan G, Xu B, et al. Cerium oxide nanoparticles reduce x-ray irradiation-induced damage to the immune cells by upregulation of superoxide dismutase and glutathione peroxidase. Nanoscience Nanotechnol Letters 2019; 11(10):1464-9.
- Benameur L, Auffan M, Cassien M, Liu W, Culcasi M, Rahmouni H, et al. DNA damage and oxidative stress induced by CeO2 nanoparticles in human dermal fibroblasts: Evidence of a clastogenic effect as a mechanism of genotoxicity. Nanotoxicol 2015; 9(6):696-705. doi: 10.3109/17435390.2014.968889.
- Estevez AY, Erlichman JS. Cerium oxide nanoparticles for the treatment of neurological oxidative stress diseases. Oxidative stress: Diagnostics, Prevention Therapy: ACS Publications 2011; 255-88.
- Saravanakumar K, Sathiyaseelan A, Mariadoss AVA, Wang MH. Antioxidant and antidiabetic properties of biocompatible ceria oxide (CeO2) nanoparticles in mouse fibroblast NIH3T3 and insulin resistant HepG2 cells. Ceramics Int 2021; 47(6):8618-26.
- Hirst SM, Peairs AD, Gogal Jr R, Seal S, Reilly CM. Cerium oxide nanoparticles decrease inflammation in J774 cells. Wiley online library; 2008.
- Nithya P, Sundrarajan M. Ionic liquid functionalised biogenic synthesis of AgAu bimetal doped CeO2 nanoparticles from Justicia adhatoda for pharmaceutical applications: Antibacterial and anti-cancer activities. J Photochemistry Photobiol Biol 2020; 202:111706. doi: 10.1016/j.jphotobiol.2019. 11170
- Chen G, Xu Y. Biosynthesis of cerium oxide nanoparticles and their effect on lipopolysaccharide (LPS) induced sepsis mortality and associated hepatic dysfunction in male sprague dawley rats. Mater Sci Eng C Mater Biol Appl 2018; 83:148-53.
- Williams AM, Bhatti UF, Brown JF, Biesterveld BE, Kathawate RG, Graham NJ, et al. Early single-dose treatment with exosomes provides neuroprotection and improves blood-brain barrier integrity in swine model of traumatic brain injury and hemorrhagic shock. J Trauma Acute Care Surg 2020; 88(2):207-18. doi: 10.1097/TA.0000000000002563.
- Hegazy MAE, Maklad HM, Abd Elmonsif DA, Elnozhy FY, Alqubiea MA, Alenezi FA. The possible role of cerium oxide (CeO2) nanoparticles in prevention of neurobehavioral and neurochemical changes in 6-hydroxydopamineinduced parkinsonian disease. Alexandria J Med 2017; 53(4):351-60.
- Wagner N, Dieteren S, Franz N, Köhler K, Mörs K, Nicin L, et al. Ethyl pyruvate ameliorates hepatic injury following blunt chest trauma and hemorrhagic shock by reducing local inflammation, NF-kappaB activation and HMGB1 release. PloS One 2018; 13(2):e0192171. doi: 10.1371/journal.pone. 0192171.
- Chen G, Song X, Wang B, You G, Zhao J, Xia S, et al. Carboxyfullerene nanoparticles alleviate acute hepatic injury in severe hemorrhagic shock. Biomaterials 2017; 112:72-81. doi: 10.1016/j.biomaterials.2016.10.022.
- Liu FC, Chaudry IH, Yu HP. Hepatoprotective effects of corilagin following hemorrhagic shock are through akt-dependent pathway. Shock 2017; 47(3):346.doi:10.1097/SHK. 0000000000000736.
- Chen G, You G, Wang Y, Lu M, Cheng W, Yang J, et al. Effects of synthetic colloids on oxidative stress and inflammatory response in hemorrhagic shock: Comparison of hydroxyethyl starch 130/0.4, hydroxyethyl starch 200/0.5, and succinylated gelatin. Critical Care 2013; 17(4):1-9. doi: 10.1186/cc12820.
- Tsai YF, Yu HP, Chung PJ, Leu YL, Kuo LM, Chen CY, et al. Osthol attenuates neutrophilic oxidative stress and hemorrhagic shock-induced lung injury via inhibition of phosphodiesterase 4. Free Radic Biol Med 2015; 89:387-400. doi: 10.1016/j.freeradbiomed.2015.08.008.
- Jiang H, Liu J, Xu Z, Zheng C. Efficacy of different fluid resuscitation methods on coagulation function of rats with traumatic hemorrhagic shock. J Surg Res 2021; 260:259-66. doi: 10.1016/j.jss.2020.11.014.
- Ding N, Chen G, Hoffman R, Loughran PA, Sodhi CP, Hackam DJ, et al. Toll-like receptor 4 regulates platelet function and contributes to coagulation abnormality and organ injury in hemorrhagic shock and resuscitation. Circ Cardiovasc Genet 2014; 7(5):615-24. doi: 10.1161/CIRCGENETICS.113.000 398.
- Niu J, Azfer A, Rogers LM, Wang X, Kolattukudy PE. Cardioprotective effects of cerium oxide nanoparticles in a transgenic murine model of cardiomyopathy. Cardiovascular Res 2007; 73(3):549-59. doi: 10.1016/j.cardiores.2006.11.031.
- Gojova A, Lee JT, Jung HS, Guo B, Barakat AI, Kennedy IM. Effect of cerium oxide nanoparticles on inflammation in vascular endothelial cells. Inhal Toxicol 2009; 21(sup1):123-30. doi: 10.1080/08958370902942582.
- Zhao S, Li Y, Liu Q, Li S, Cheng Y, Cheng C, et al. An orally administered CeO2@ montmorillonite nanozyme targets inflammation for inflammatory bowel disease therapy. Advan Funct Mater 2020; 30(45):2004692.
- Wu L, Liu G, Wang W, Liu R, Liao L, Cheng N, et al. Cyclodextrin-modified CeO2 nanoparticles as a multifunctional nanozyme for combinational therapy of psoriasis. Int J Nano Med 2020; 15:2515. doi: 10.2147/IJN.S246783.
- Tsai SM, Duran-Robles E, Goshia T, Mesina M, Garcia C, Young J, et al. CeO2 nanoparticles attenuate airway mucus secretion induced by TiO2 nanoparticles. Sci Total Envir 2018; 631:262-9. doi: 10.1016/j.scitotenv.2018.03.001.
- Li H, Xia P, Pan S, Qi Z, Fu C, Yu Z, et al. The advances of ceria nanoparticles for biomedical applications in orthopaedics. Int J Nanomedicine 2020; 15:7199-7214. doi: 10.2147/IJN.S270229.