Association between Insulin Resistance and Cognitive Impairment
By Mingyue Chen1, Min Zhang1, Shenglin Wang1, Xiaomi Ding1, Yujun Lee2, Guohui Jiang1Affiliations
doi: 10.29271/jcpsp.2022.02.202ABSTRACT
The objective of the review was to assess the relationship between insulin resistance and cognitive impairment. Medline, Embase, Web of Science and Cochrane Library were searched. Two independent authors selected studies and extracted data. Quality of included studies was assessed by NOS (Newcastle-Ottawa quality assessment scale). A random-effects model with its 95% confidence intervals (CIs) was considered for meta-analysis. Eight articles including 1,399 subjects were included in this meta-analysis. The article showed a negative association between insulin resistance and cognition (R = − 0.262; 95% CI−0.309, − 0.215). There is evidence that insulin resistance may be a mechanism of cognitive impairment.
Key Words: Insulin resistance, Insulin, Cognition, Cognitive impairment, Systematic review, Alzheimer’s disease.
INTRODUCTION
Cognitive impairment generally refers to the degree of cognitive impairment caused by various causes, including mild cognitive impairment, vascular dementia, which causes by stroke, and Alzheimer's disease (AD) etc. Symptoms of cognitive impairment may include learning and memory impairment, also accompanied with loss of speech, use, recognition, and behaviour. Alzheimer's Disease International (ADI) report estimated that in 2019, over 50 million people were living with dementia globally, and a figure set to increase to 152 million by 2050.1 Meanwhile, research by Jianping’s team shows that among the population over 60 years old in China, the number of dementia patients is 10-11 million, and more than 60% of dementia patients suffer from AD.2
Moreover, AD is the number one neurodegenerative disease of the central nervous system. Because of the multiple causative factors of cognitive impairment and the severity of the disease, coupled with the fact that there is no effective prevention or treatment, it is clinically important to study its mechanisms.3-5
At present, there has been a great deal of research on the mechanism of cognitive impairment including oxidative stress, inflammation, and insulin resistance.6-8 Insulin resistance is one of the most studied mechanisms in recent years, which can be defined as decreased sensitivity of brain cells to insulin.9,10 Many studies have showed that insulin affects physiological and pathological processes in the body through its two main effector pathways: The MAPK pathway and the phosphatidylinositol (PI) 3-kinase (PI3K) / Akt pathway, which often involves neuronal survival, synaptic maintenance, dendritic development, cognition, neural circuit formation, BBB transporter expression/localisation and so on.11-15 Insulin receptors are present in many areas of the brain, such as the cerebral cortex, choroid plexus, hypothalamus, and the hippocampus, the area most associated with cognition.16 Current study confirms that disorders of insulin and insulin receptors (IRs) signalling, play a crucial pathophysiological role in the onset and progression of central nervous system disorders such as neurodegenerative diseases and neuropsychiatric disorders, especially in the development of cognitive impairment.17-20 Alzheimer's disease is also known as type 3 diabetes.
The purpose of this systematic review was to evaluate the association between insulin resistance and cognitive impairment, and to provide rigorous study results.
METHODOLOGY
This systematic review and meta-analysis was conducted in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standard.21
Table I: Characteristics of the studies included in this meta-analysis.|
Author |
Country |
Study design |
N |
Population |
Age(years) mean±SD(range) |
Cognitive rating scale |
Insulin resistance measurement |
R value |
P value |
Convert R value |
|
Lin, 2019 |
China |
Case-control |
133 |
Diabetics |
45-75 |
MOCA |
HOMA-IR |
-0.239 |
- |
- |
|
Kong, 2018 |
Korea |
Cohort |
422 |
Elderly people |
>45 |
K-MMSE |
HOMA-IR |
-0.155 |
- |
- |
|
Ekblad, 2018 |
Finland |
Cohort |
60 |
Elderly volunteers without dementia |
55.4 |
CERAD |
HOMA-IR |
- |
<0.0001 |
-0.064 |
|
Yang, 2017 |
China |
Case-control |
282 |
PD patients |
70.23±7.54 |
MOCA |
HOMA-IR |
-0.027 |
- |
- |
|
Sun, 2016 |
China |
Case-control |
75 |
patients with MCI |
40-80 |
MOCA |
HOMA-IR |
-0.619 |
- |
- |
|
Hishikaw, 2015 |
Japan |
Case-control |
182 |
Diabetics |
64.7±18 |
MOCA |
HOMA-IR |
- |
<0.05 |
-0.146 |
|
Zhong, 2012 |
China |
Case-control |
328 |
Normal elderly |
>70 |
MMSE |
HOMA-IR |
-0.226 |
- |
- |
|
Rasgo, 2011 |
United States |
Longitudinal |
50 |
Postmenopau- sal women |
50-65 |
MMSE |
HOMA-IR |
- |
0.08 |
-0.232 |
|
N: Number of participants; SD: Standard deviation; PD: Parkinson's disease; MCI: Mild cognitive impairment; CERAD: Finnish version of the consortium to establish a registry for Alzheimer’s disease; K-MMSE: Korean mini-mental status examination. |
||||||||||
Table II: Quality evaluation of cohort studies.
|
Author |
Year |
Representativeness of exposed cohort |
Selection of nonexposed cohort |
Ascertainment of exposure |
Demonstration that outcome of interest was not present at start of study |
Comparability of cohorts on basis of design or analysis |
Assessment of outcome |
Was follow-up long enough for outcome to occur |
Adequacy of follow up of cohorts |
Total points |
|
Kong |
2018 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
8 |
|
Ekblad |
2018 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
7 |
Table III: Quality evaluation of case-control studies.
|
Author |
Year |
Is the case definition adequate? |
Representativeness of the cases |
Selection of Controls |
Definition of Controls |
Comparability of cases and controls on the basis of the design or analysis |
Ascertainment of exposure |
Same method of ascertain-ment for cases and controls |
Non-Resp-onse rate |
Total points |
|
Lin |
2019 |
1 |
1 |
0 |
1 |
2 |
1 |
1 |
0 |
6 |
|
Yang |
2017 |
1 |
1 |
0 |
1 |
1 |
1 |
1 |
0 |
6 |
|
Sun |
2016 |
1 |
1 |
0 |
1 |
1 |
1 |
1 |
1 |
7 |
|
Hishikw |
2015 |
1 |
1 |
0 |
1 |
1 |
1 |
1 |
1 |
7 |
|
Zhong |
2012 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
7 |
|
Rasgon |
2011 |
1 |
1 |
1 |
1 |
0 |
1 |
1 |
1 |
6 |
PubMed, Embase, Web of Science, and Cochrane Library were searched without language restriction from January 2010 until December 2019 by three authors. MeSH and Emtree terms were used in the search equation. Search terms were insulin resistance, cognition, cognitions. Furthermore, bibliographic references list of included articles was also searched manually in order to identify other articles that meet the inclusion criteria. The details are given in Table I.
The criteria for inclusion were: measuring the insulin resistance index by HOMA-IR; cognitive rating scale be a comprehensive scale such as MMSE or MOCA or CERAD; and p or r values availability. The criteria for exclusion were: animal or basic science research; non-availability of full text; result data; not extractable; summaries; and letters.
From each included article, two authors extracted study characteristics (title, name of the first author, publication year, inclusion crowd); sample characteristics (age, gender etc.); cognitive rating scale and the evaluation index of insulin resistance; p or r- value. P-values to r-values were converted by Eq. Any disagreement was resolved through communication. The Newcastle-Ottawa quality assessment scale was used to evaluate the methodological quality of case-control studies and cohort studies. A NOS score of more than 3 is considered qualified, and the higher the score, the higher the quality (Tables II & III).22
R-value and 95% confidence interval (CI) were calculated to evaluate the overall degree of relevance. Heterogeneity among the included studies was evaluated by the Chi-based Q statistic. If there was a significant heterogeneity (I2 >50% or p >0.10),23 the random-effects model was performed to pool the outcomes; otherwise, the fixed effect model was applied. Sensitivity analysis was used to discover the source of heterogeneity and tried to explain it. All statistical analyses were conducted by comprehensive meta-analysis software 3.0 (CMA 3.0).
RESULTS
Figure 1 shows the entire search process. By searching four databases, a total of 5,522 documents were obtained. After removing duplicates and excluding documents according to the selection criteria, eight documents were included.
There were 1,399 participants in the eight documents, each with a different condition and a wide age range that was unevenly distributed (Table I). From Table I, it can be seen that one literature was based on patients with cognitive impairment, four on cognitively normal people, two on diabetics, and one on people with Parkinson's disease. Overall, differences in the study population and its age may lead to high heterogeneity.
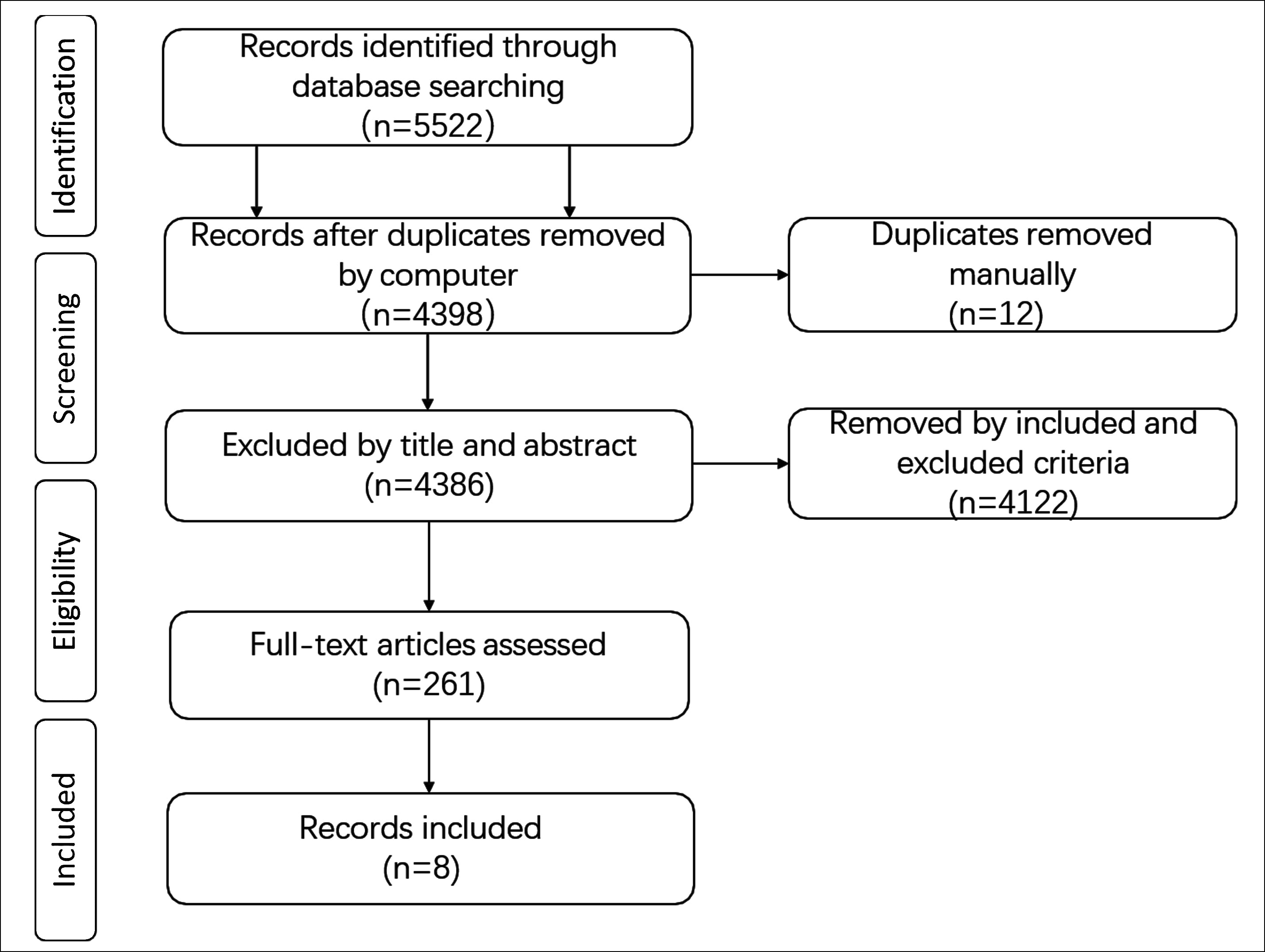 Figure 1: Flow diagram of bibliographic retrievals and results.
Figure 1: Flow diagram of bibliographic retrievals and results.
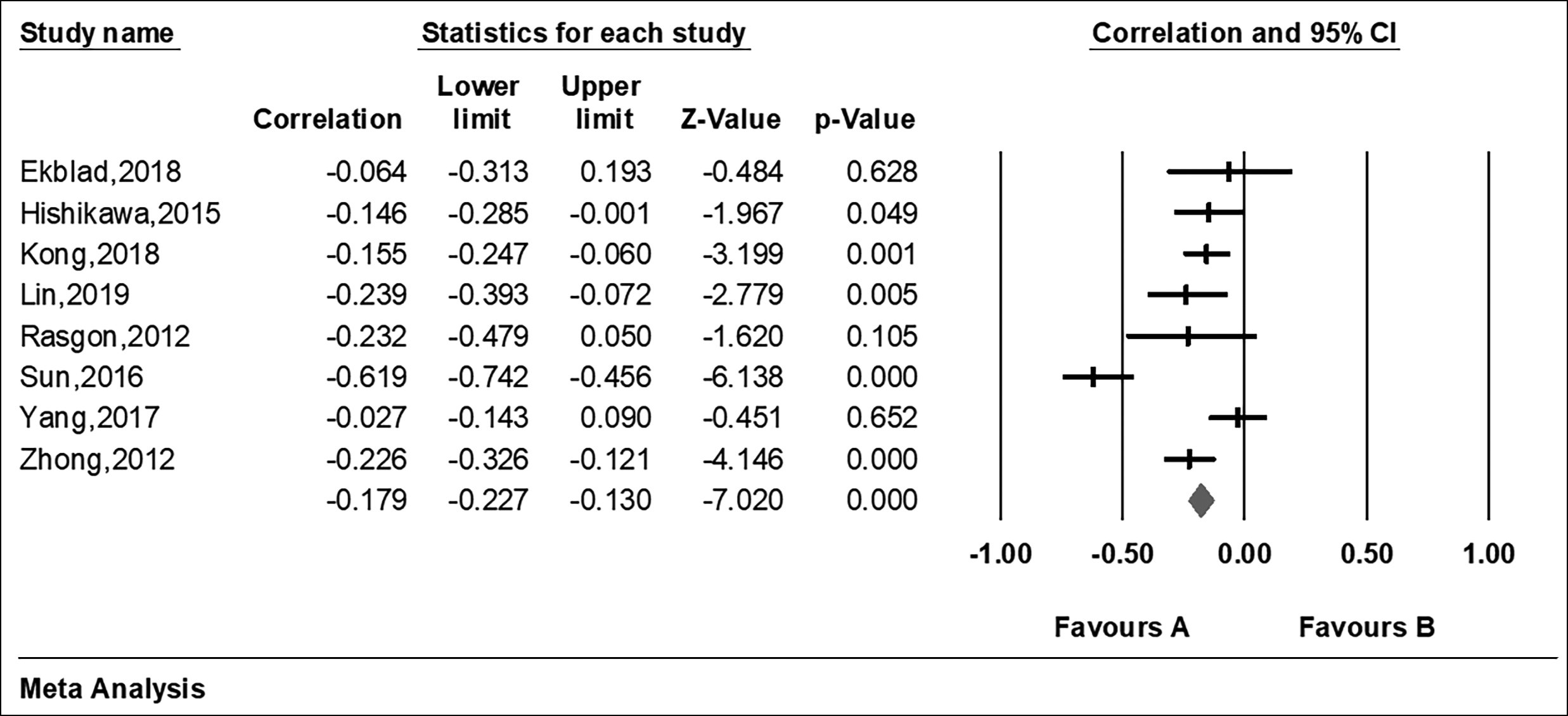 Figure 2: Forest plots of meta-analysis of the included studies on the association between IR and cognition impairment parameters.
Figure 2: Forest plots of meta-analysis of the included studies on the association between IR and cognition impairment parameters.
The ‘R’ or ‘p’ value in the eight articles were extracted to evaluate the relationship between insulin resistance and cognitive impairment. At the same time, the HOMA-IR and the cognitive assessment scale of each article were extracted to assess for insulin resistance and cognitive impairment respectively (Table I). From Figure 2, the forest plot of the R values was based on a fixed-effect model , and showed that cognition is negatively associated with insulin resistance after the merger effect volume (R = −0.179; 95% CI −0.227, −0.130). The authors tested heterogeneity of R values between the studies (I2 = 77.029% >50%, p <0.001), suggesting that there was statistically significant heterogeneity among included studies (Figure 3). Sensitivity analysis was then performed in order to determine the source of heterogeneity. From Figures 4 and 5, after removing a single study (Sun, 2016), the heterogeneity between the rest studies has changed (I2 = 26.929% <50%, p = 0.223), which is acceptable (Figures 4 & 5).
DISCUSSION
This systematic review and meta-analysis included eight articles with 1,399 participants. Consistent with the expectations, the results demonstrated that there is a negative correlation between cognitive function and insulin resistance, which is statistically significant.
As mentioned above, heterogeneity becomes acceptable after removing an outlier. The population included in that study was diabetic patients, and the population was divided into groups with and without MCI, based on MOCA scores. The correlation between HOMA-IR and MOCA scores was performed only in the MCI group; whereas, in the rest of the literature, the correlation between the two was performed in all included populations, which may lead to a large difference in the correlation coefficients. In addition, the sample size of people included in the MCI group was small, so the results may lead to bias.
Insulin resistance refers to a decrease in the sensitivity and responsiveness of insulin target organs or tissues to insulin, resulting in a lower than normal amount of insulin producing biological effects and an increase in fasting plasma insulin levels to maintain normal insulin action.23 Studies have shown that insulin resistance is associated with increased nutrient-derived toxic metabolites (DAG, ceramides, acylcarnitines, circulating branched-chain amino acids), overdrive of nutrient utilisation processes (endoplasmic reticulum stress and oxidative stress), and response to nutrient stress-mediated cytotoxicity (inflammatory response).24 Of these, the inflammatory response is also an important mechanism leading to cognitive impairment.25 The literature suggests that neuroinflammation is an important process in neurodegeneration in AD, which is involved in a vicious cycle of amyloid deposition, neuronal damage, entanglement formation, and death.26 Heneka et al. demonstrated that Aβ-induced NLRP3 inflammasome activation promotes AD progression by mediating deleterious chronic inflammatory tissue responses, that its activation-induced inflammatory mediators may be involved in synaptic dysfunction, cognitive deficits, and limitations in beneficial microglial clearance; and that blocking NLRP3 inflammasomes has the potential to effectively intervene in the progress in AD.27 Another study confirmed that NLRP3 inflammatory microsomes are also involved in Aβ-induced Tau protein phosphorylation.28 The use of NSAIDs ibuprofen in an AD transgenic (Tg) mouse model has been shown to reduce Aβ deposition and astrocyte and microglial cell activation and Tau protein phosphorylation in the hippocampus.29,30
In addition, several large clinical studies have confirmed that some inflammatory factors such as elevated sensitivity to CRP, TNF-α, and IL-6 are associated with cognitive decline.31-33
This meta-analysis provided (yielded) evidence of a linear negative correlation between insulin resistance and cognitive function. As mentioned earlier, the mechanisms of both insulin resistance and cognitive dysfunction include inflammatory responses, so inflammatory responses may be one of the reasons why there is a negative correlation between insulin resistance and cognitive function. According to one study, neuroinflammation increases insulin resistance by increasing serine phosphorylation in the IRS, and increased insulin resistance can lead to cognitive decline in the brain.34,35
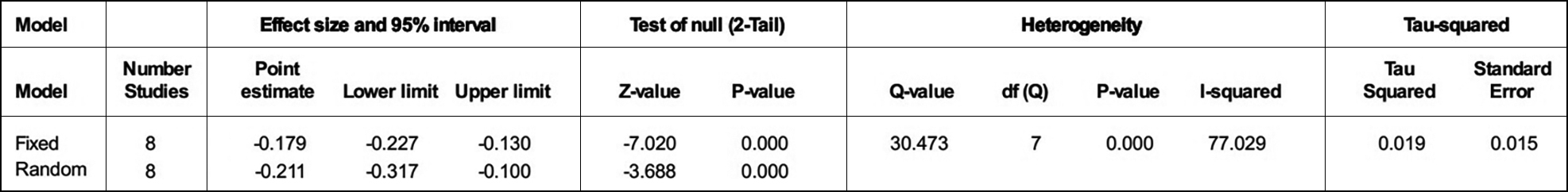 Figure 3: Heterogeneity test.
Figure 3: Heterogeneity test.
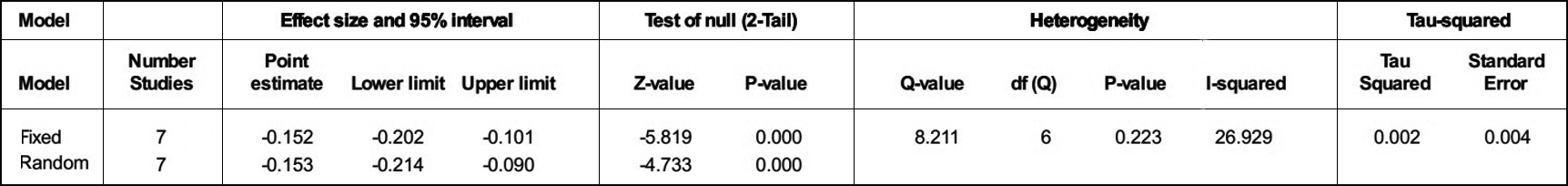 Figure 4: Heterogeneity test after removal of the more heterogeneous literature.
Figure 4: Heterogeneity test after removal of the more heterogeneous literature.
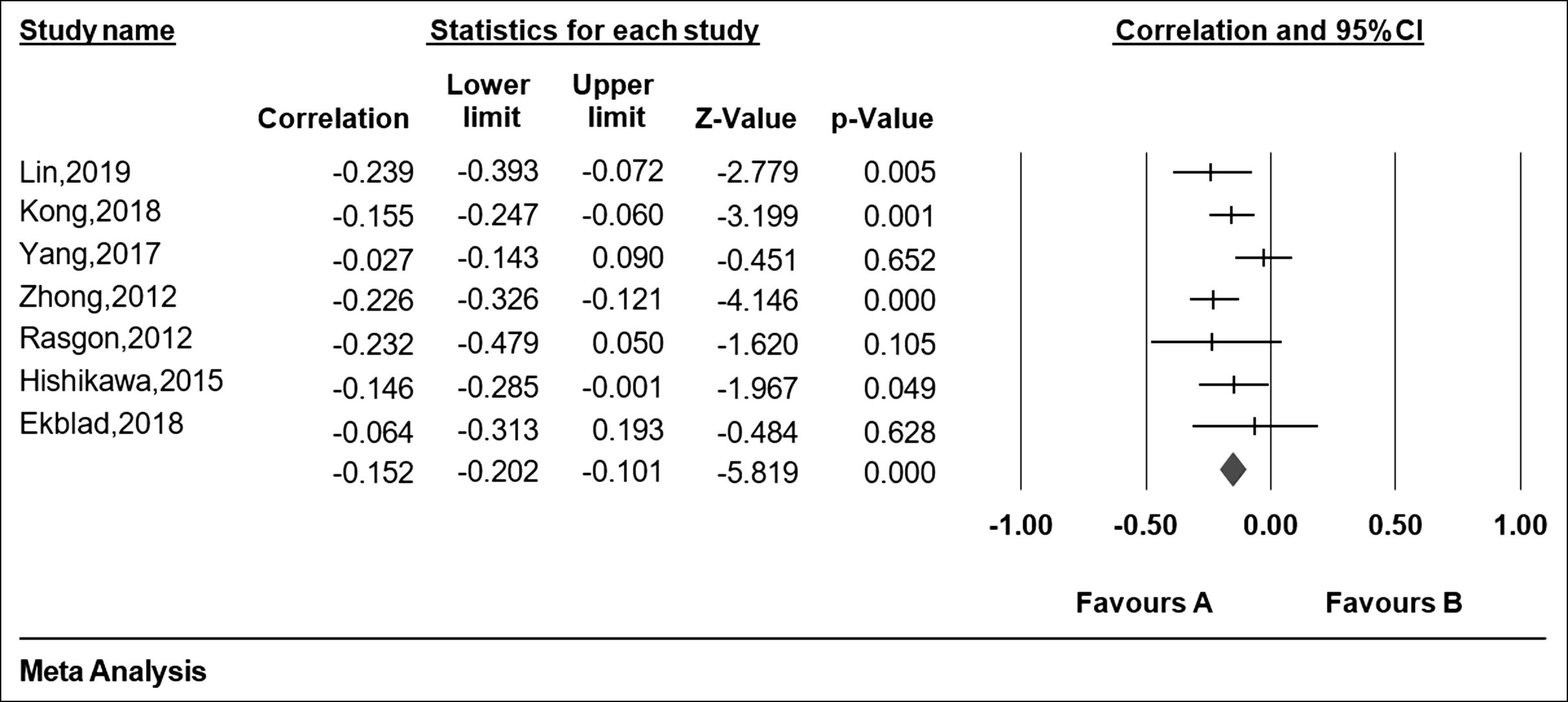 Figure 5: Forest plots of meta-analysis of after removal of the more heterogeneous literature.
Figure 5: Forest plots of meta-analysis of after removal of the more heterogeneous literature.
An animal study showed that yeast polysaccharides can cause impaired insulin signalling through inflammatory pathway, leading to insulin resistance and subsequent cognitive decline.36
Insulin has complex effects on metabolism, cell proliferation and differentiation, with the main effects being the promotion of tissue glucose utilisation, fatty acid uptake and inhibition of glucose production.37 In addition to its peripheral effects, insulin also plays a role in the central nervous system. Hippocampal neurons are particularly susceptible to altered insulin sensitivity.38 It is established that insulin-mediated activation of the PI3K/AKT pathway enhances glucose and energy metabolism in the brain.39 It has been demonstrated that AD has decreased sensitivity to insulin signalling in the IRS-1/PI3K pathway; and thus insulin resistance occurs.40 In addition, decreased activation of PI3K/AKT can lead to increased activation of GSK-3β, resulting in tau protein phosphorylation as well as increased Aβ deposition.41 The increased Aβ oligomers may inhibit insulin signalling, which may lead to a vicious cycle.42 Most importantly, a positive correlation between increased GSK-3βmRNA gene expression and tau protein phosphorylation has been reported.43 In addition, Hui et al. demonstrated that intranasal administration of insulin enhances cognitive function and hippocampal neurogenesis in mice, with brain insulin resistance by activating the IRS-1-PI3K-Akt pathway.44
However, there are still some shortcomings. Firstly, the time limit of the literature search is between 2010-2019; thus, some valuable experiments may be missed. Secondly, the database contains mostly literature in English; and non-English literature were not considered. Besides, there is a lack of data, necessary to further discuss the sources of heterogeneity.
CONCLUSION
Overall, this is the first meta-analysis of whether there is a correlation between insulin resistance and cognitive impairment, providing high-level evidence for the clinical use of insulin in cognitive impairment as well as clinical and basic research. In summary, insulin resistance is negatively correlated with cognition. This evidence contributes to further understanding of the mechanisms by which cognitive impairment occurs; and future therapies could consider interventions, targeting insulin resistance in cognitive impairment.
ACKNOWLEDGMENTS:
This work was supported by funding from the National Natural Science Foundation of China (No. 81971220), the Science and Technology Department of Sichuan Province (No. 2018JY0236), and the Department of Science and Technology of Sichuan Province (No. 2018JY0279).
CONFLICT OF INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
MC: Writing-Original draft preparation and Methodology.
SW, MZ: Methodology and formal analysis.
XD: Methodology and visualisation.
YL, GJ: Conceptualisation, project administration, funding acquisition, supervision.
REFERENCES
- Abbassi R, Johns TG, Kassiou M, Munoz L. DYRK1A in neurodegeneration and cancer: Molecular basis and clinical implications. Pharmacol Ther 2015; 151:87-98. doi: 10.1016/j.pharmthera.2015.03.004.
- Jia J, Wei C, Chen S, Li F, Tang Y, Qin W, et al. The cost of Alzheimer's disease in China and re-estimation of costs worldwide. Alzheimer's Dement 2018; 14(4):483-91. doi: 10.1016/j.jalz.2017.12.006.
- Patnaik N. Cure for Alzheimer's Disease. World J Neuroence 2015; 5(5):328-30.
- Cummings JL, Morstorf T, Zhong K. Alzheimer's disease drug-development pipeline: Few candidates, frequent failures. Alzheimer's Res Ther 2014; 6(4):37. doi: 10.1186/alzrt269.
- Loera-Valencia R, Cedazo-Minguez A, Kenigsberg PA, Page G, Duarte AI, Giusti P, et al. Current and emerging avenues for Alzheimer's disease drug targets. J Intern Med 2019; 286(4):398-437. doi: 10.1111/joim.12959.
- Butterfield DA, Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci 2019; 20(3):148-60. doi: 10.1038/s41583- 019-0132-6.
- Liu L, Chan C. The role of inflammasome in Alzheimer's disease. Ageing Res Rev 2014; 15:6-15. doi: 10.1016/j.arr.2013.12.007.
- de la Monte SM. Brain insulin resistance and deficiency as therapeutic targets in Alzheimer's disease. Curr Alzheimer Res 2012; 9(1):35-66. doi: 10.2174/156720512799015037.
- Mielke JG, Taghibiglou C, Liu L, Zhang Y, Jia Z, Adeli K, et al. A biochemical and functional characterization of diet-induced brain insulin resistance. J Neurochem 2005; 93(6):1568-78. doi: 10.1111/j.1471-4159.2005.03155.x.
- Kulas JA, Weigel TK, Ferris HA. Insulin resistance and impaired lipid metabolism as a potential link between diabetes and Alzheimer's disease. Drug Dev Res 2020; 81(2):194-205. doi: 10.1002/ddr.21643.
- Banks WA, Owen JB, Erickson MA. Insulin in the brain: There and back again. Pharmacol Ther 2012; 136(1):82-93. doi: 10.1016/j.pharmthera.2012.07.006.
- Banks WA. The source of cerebral insulin. Eur J Pharmacol 2004; 490(1-3):5-12. doi: 10.1016/j.ejphar.2004.02.040.
- Schulingkamp RJ, Pagano TC, Hung D, Raffa RB. Insulin receptors and insulin action in the brain: Review and clinical implications. Neurosci Biobehav Rev 2000; 24(8):855-72. doi: 10.1016/s0149-7634(00)00040-3.
- Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, et al. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus 2008; 18(11):1085-8. doi: 10.1002/ hipo.20470.
- Rhea EM, Raber J, Banks WA. ApoE and cerebral insulin: Trafficking, receptors, and resistance. Neurobiol Dis 2020; 137:104755. doi: 10.1016/j.nbd.2020.104755.
- Hill JM, Lesniak MA, Pert CB, Roth J. Autoradiographic localisation of insulin receptors in rat brain: Prominence in olfactory and limbic areas. Neuroscience 1986; 17(4):1127-38. doi: 10.1016/0306-4522(86)90082-5.
- Ghasemi R, Dargahi L, Haeri A, Moosavi M, Mohamed Z, Ahmadiani A. Brain insulin dysregulation: Implication for neurological and neuropsychiatric disorders. Mol Neurobiol 2013; 47(3):1045-65. doi: 10.1007/s12035-013-8404-z.
- Benarroch EE. Insulin-like growth factors in the brain and their potential clinical implications. Neurology 2012; 79(21):2148-53. doi: 10.1212/WNL.0b013e3182752eef.
- de la Monte SM, Wands JR. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: Relevance to Alzheimer's disease. J Alzheimer's Dis 2005; 7(1):45-61. doi: 10.3233/jad- 2005-7106.
- Chan ES, Chen C, Soong TW, Wong BS. Differential binding of human ApoE Isoforms to insulin receptor is associated with aberrant insulin signaling in AD brain samples. Neuromolecular Med 2018; 20(1):124-32. doi: 10.1007/s12017- 018-8480-3.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med 2009; 6(7):e1000097. doi: 10.1371/journal.pmed.1000097.
- Stang A. Critical evaluation of the Newcastle-ottawa scale for the assessment of the quality of nonrandomised studies in meta-analyses. Eur J Epidemiol 2010; 25(9):603-5. doi: 10.1007/s10654-010-9491-z.
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327(7414): 557-60. doi: 10.1136/bmj.327.7414.557.
- Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev 2018; 98(4):2133-23. doi: 10.1152/physrev.00063.2017.
- Zhou J, Zhang Z, Zhou H, Qian G. Diabetic cognitive dysfunction: From bench to clinic. CurrMed Chem 2020; 27(19):3151-67. doi: 10.2174/1871530319666190206 225635.
- Calsolaro V, Edison P. Neuroinflammation in Alzheimer's disease: Current evidence and future directions. Alzheimer's Dement 2016; 12(6):719-32. doi: 10.1016/j.jalz.2016. 02.010.
- Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, et al. NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature 2013; 493(7434):674-8. doi: 10.1038/nature11729.
- Ising C, Venegas C, Zhang S, Scheiblich H, Schmidt SV, Vieira-Saecker A, et al. NLRP3 inflammasome activation drives tau pathology. Nature 2019; 575(7784):669-73. doi: 10.1038/s41586-019-1769-z.
- McGeer PL, McGeer EG. NSAIDs and Alzheimer disease: Epidemiological, animal model and clinical studies. Neurobiol Aging 2007; 28(5):639-47. doi: 10.1016/j.neurobiolaging.2006.03.013.
- Choi SH, Aid S, Caracciolo L, Minami SS, Niikura T, Matsuoka Y, et al. Cyclooxygenase-1 inhibition reduces amyloid pathology and improves memory deficits in a mouse model of Alzheimer's disease. J Neurochem 2013; 124(1):59-68. doi: 10.1111/jnc.12059.
- Noble JM, Manly JJ, Schupf N, Tang MX, Mayeux R, Luchsinger JA. Association of C-reactive protein with cognitive impairment. Arch Neurol 2010; 67(1):87-92. doi: 10.1001/archneurol.2009.308.
- Yaffe K, Kanaya A, Lindquist K, Simonsick EM, Harris T, Shorr RI, et al. The metabolic syndrome, inflammation, and risk of cognitive decline. JAMA 2004; 292(18):2237-42. doi: 10.1001/jama.292.18.2237.
- Holmes C, Cunningham C, Zotova E, Woolford J, Dean C, Kerr S, et al. Systemic inflammation and disease progression in Alzheimer disease. Neurology 2009; 73(10):768-74. doi: 10.1212/WNL.0b013e3181b6bb95.
- Xu M, Huang H, Mo X, Zhu Y, Chen X, Li X, et al. Quercetin-3-O-Glucuronide Alleviates Cognitive Deficit and Toxicity in Aβ(1-42) -Induced AD-Like Mice and SH-SY5Y Cells. Mol Nut Food Res 2021; 65(6):e2000660. doi: 10.1002/mnfr.202000660.
- Lourenco MV, Clarke JR, Frozza RL, Bomfim TR, Forny-Germano L, Batista AF, et al. TNF-α mediates PKR-dependent memory impairment and brain IRS-1 inhibition induced by Alzheimer's β-amyloid oligomers in mice and monkeys. Cell Metab 2013; 18(6):831-43. doi: 10.1016/j.cmet.2013. 11.002.
- Ahuja S, Uniyal A, Akhtar A, Sah SP. Alpha lipoic acid and metformin alleviates experimentally induced insulin resistance and cognitive deficit by modulation of TLR2 signalling. Pharmacol Rep 2019; 71(4):614-23. doi: 10.1016/j.pharep. 2019.02.016.
- Haeusler RA, McGraw TE, Accili D. Biochemical and cellular properties of insulin receptor signalling. Nat Rev Mol Cell Biol 2018; 19(1):31-44. doi: 10.1038/nrm.2017.89.
- Fehm HL, Kern W, Peters A. The selfish brain: Competition for energy resources. Progin Brain Res 2006; 153:129-40. doi: 10.1016/S0079-6123(06)53007-9.
- Akhtar A, Dhaliwal J, Saroj P, Uniyal A, Bishnoi M, Sah SP. Chromium picolinate attenuates cognitive deficit in ICV-STZ rat paradigm of sporadic Alzheimer's-like dementia via targeting neuroinflammatory and IRS-1/PI3K/AKT/GSK-3β pathway. Inflammopharmacol 2020; 28(2):385-400. doi: 10.1007/s10787-019-00681-7.
- Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, et al. Demonstrated brain insulin resistance in Alzheimer's disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest 2012; 122(4):1316-38. doi: 10.1172/JCI59903.
- Baki L, Shioi J, Wen P, Shao Z, Schwarzman A, Gama-Sosa M, et al. PS1 activates PI3K thus inhibiting GSK-3 activity and tau overphosphorylation: Effects of FAD mutations. EMBO J 2004; 23(13):2586-96. doi: 10.1038/sj.emboj.7600251
- Liang H, Nie J, Van Skike CE, Valentine JM, Orr ME. Mammalian target of rapamycin at the crossroad between Alzheimer's Disease and diabetes. Advan Exp Med Biol 2019; 1128:185-225. doi: 10.1007/978-981-13-3540-2_10.
- Del Pino J, Zeballos G, Anadón MJ, Moyano P, Díaz MJ, García JM, et al. Cadmium-induced cell death of basal forebrain cholinergic neurons mediated by muscarinic M1 receptor blockade, increase in GSK-3β enzyme, β-amyloid and tau protein levels. Arch Toxicol 2016; 90(5):1081-92. doi: 10.1007/s00204-015-1540-7.
- Lv H, Tang L, Guo C, Jiang Y, Gao C, Wang Y, et al. Intranasal insulin administration may be highly effective in improving cognitive function in mice with cognitive dysfunction by reversing brain insulin resistance. Cogn Neurodyn 2020; 14(3):323-38. doi: 10.1007/s11571-020-09571-z.