Association Between Gastric Antral Cross-sectional Area and Postoperative Nausea and Vomiting in Patients Undergoing Laparoscopic Cholecystectomy
By Kai Wang, Junyu Tao, Zhangrui Hu, Zixuan Guo, Jing Li, Wenjun GuoAffiliations
doi: 10.29271/jcpsp.2023.03.249ABSTRACT
Objective: To explore the predictive value of gastric antral cross-sectional area (CSA) in the occurrence of postoperative nausea and vomiting (PONV) by analysing the association between gastric antral CSA measured by ultrasound and frequency of PONV after laparoscopic cholecystectomy.
Study Design: Observational study.
Place and Duration of the Study: Department of Anaesthesiology, The First Hospital of Wannan Medical College, Yijishan Hospital of Wannan Medical College, from October 2021 to February 2022.
Methodology: Gastric antral ultrasound (US) was performed in 266 patients undergoing laparoscopic cholecystectomy (LC) before anaesthesia induction, after it, and after surgery. The data obtained were used to evaluate the relationship between gastric antral CSA and PONV.
Results: The gastric antral CSA in Semi-recumbent decubitus (SRD) position >398.85 mm2 (AUC=0.623) highly indicated the occurrence of PONV. In addition, the subject performance characteristic curve of a binary logistics retrospective model for predicting PONV (AUC=0.805) highly indicated the occurrence of PONV.
Conclusion: Gastric US assessment of gastric antral CSA might change the current assessment model of PONV risk. This study showed that the gastric antral CSA in the SRD position after anaesthesia induction and a binary logistics retrospective model could be used to predict the occurrence of PONV, which could be helpful to adjust intraoperative and postoperative interventions and accelerate the recovery of patients.
Key Words: Postoperative nausea and vomiting, Gastric ultrasound, Gastric cross-sectional area, Perioperative period.
INTRODUCTION
Laparoscopic cholecystectomy (LC) has become the gold standard for the surgical treatment of cholelithiasis, cholecystitis, and gallbladder polyps. Although the surgical methods were improved and the surgical results were satisfactory, postoperative nausea and vomiting (PONV) was a painful side effect, occurring in 46-72%.1
The cross-sectional area (CSA) of gastric antrum could reflect the whole GV, and there was a significant positive correlation between CSA of gastric antrum and GV. Once the CSA was calculated, a linear model could be used to estimate GV.2 Gastric aspiration was found to be not a reliable tool for monitoring residual GV.3
GV of healthy fasting patients was 0.4-0.6 ml/kg, and the increase of GV was positively correlated with the incidence of PONV.4 However, there was no study on the threshold of nausea and vomiting caused by gastric antral CSA and GV. Therefore, based on the gastric antral CSA measured by US, the gastric antral CSA may be obtained to predict the occurrence of PONV. The purpose of this study was to explore the predictive value of gastric antral CSA in the occurrence of PONV by analysing the association between gastric antral CSA and PONV.
METHODOLOGY
This study was registered in the Chinese Clinical Trial Registry on October 05 2021 (ChiCTR2100051815). From October 2021 to February 2022, a total of 266 patients from Wannan Medical College Yijishan Hospital between the ages of 18 and 65 years with an American Society of Anesthesiologists (ASA) status of I-II who underwent LC under general anaesthesia (GA), were included in this clinical observational study. The exclusion criteria were pregnant women, body mass index (BMI) exceeding 28 Kg/m2, receiving radiotherapy and chemotherapy; pyloric obstruction; hypoproteinemia; patients with gastric and other history of abdominal surgery; and disturbance of gastric emptying.
All US examinations were carried out by a single operator. The US operator used a curvilinear array 2 to 5 MHz probe to examine gastric antrum of patients. According to the left hepatic lobe, superior mesenteric artery, and abdominal aorta as anatomical markers, the best ultrasonic images of the gastric antrum were obtained. The patient was in scanned supine position, then the head of the bed raised to 45° with the patient in semi-recumbent decubitus (SRD) and then the patient was assisted to the right lateral decubitus (RLD) position. The CSA of the gastric antrum was measured in the preparation room, the operating table and the bed of the postanaesthesia care unit (PACU).
The CSA of gastric antrum was calculated (CSA, in mm2, CSA = π × D1 × D2/4).5 A mathematical model for calculating GV was proposed and verified in adults, as volume (ml) = 1199.99 + 483.09 × log (gastric antrum CSA, cm²)-5.84 × age (years)-9.94 × height (cm).5 Each gastric antral CSA was measured 3 times, and the average value was recorded as the result of this measurement. The cross-sectional images of the gastric antrum at rest (gastric peristaltic interval) were obtained, and the head-tail diameter (D1) and anterior-posterior diameter (D2) of gastric antrum in different positions were measured. At the same time, the authors also used the free tracking method (FTM) formula to measure gastric antral CSA (Figure 1).
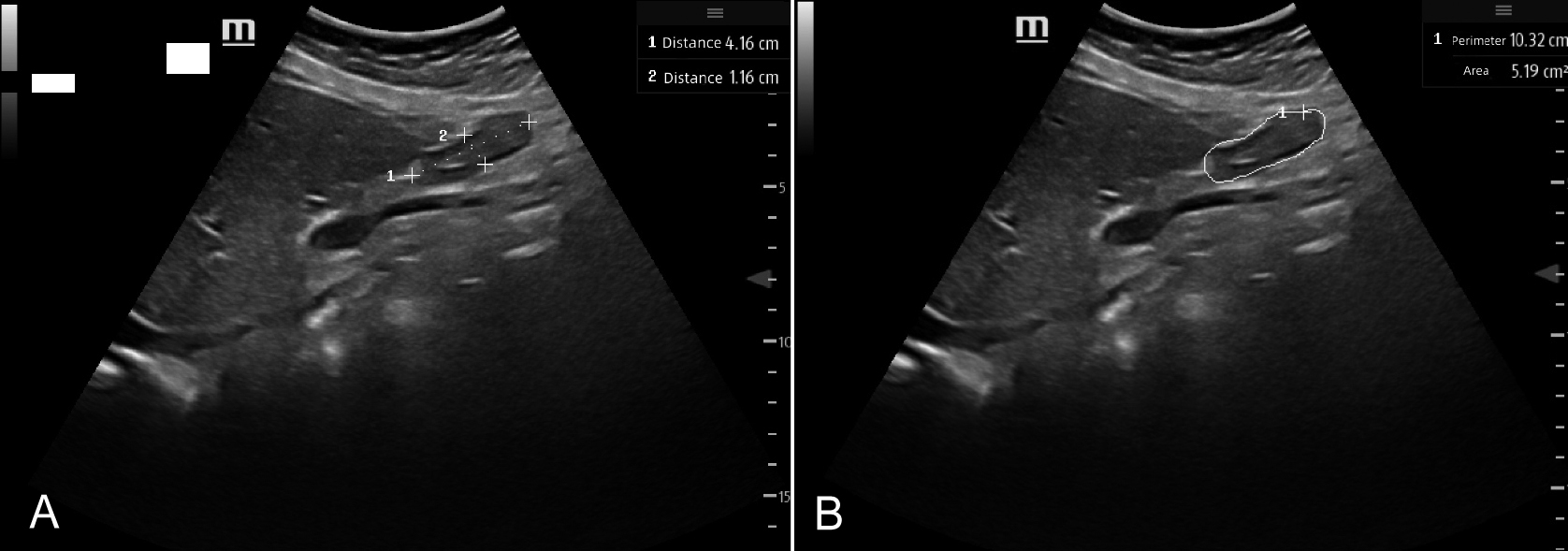 Figure 1: Two methods to measure gastric antral CSA: A US displayed the head-tail diameter (D1) and anterior-posterior diameter (D2) of gastric antrum; B US displayed a method using FTM to measure gastric antral CSA.
Figure 1: Two methods to measure gastric antral CSA: A US displayed the head-tail diameter (D1) and anterior-posterior diameter (D2) of gastric antrum; B US displayed a method using FTM to measure gastric antral CSA.
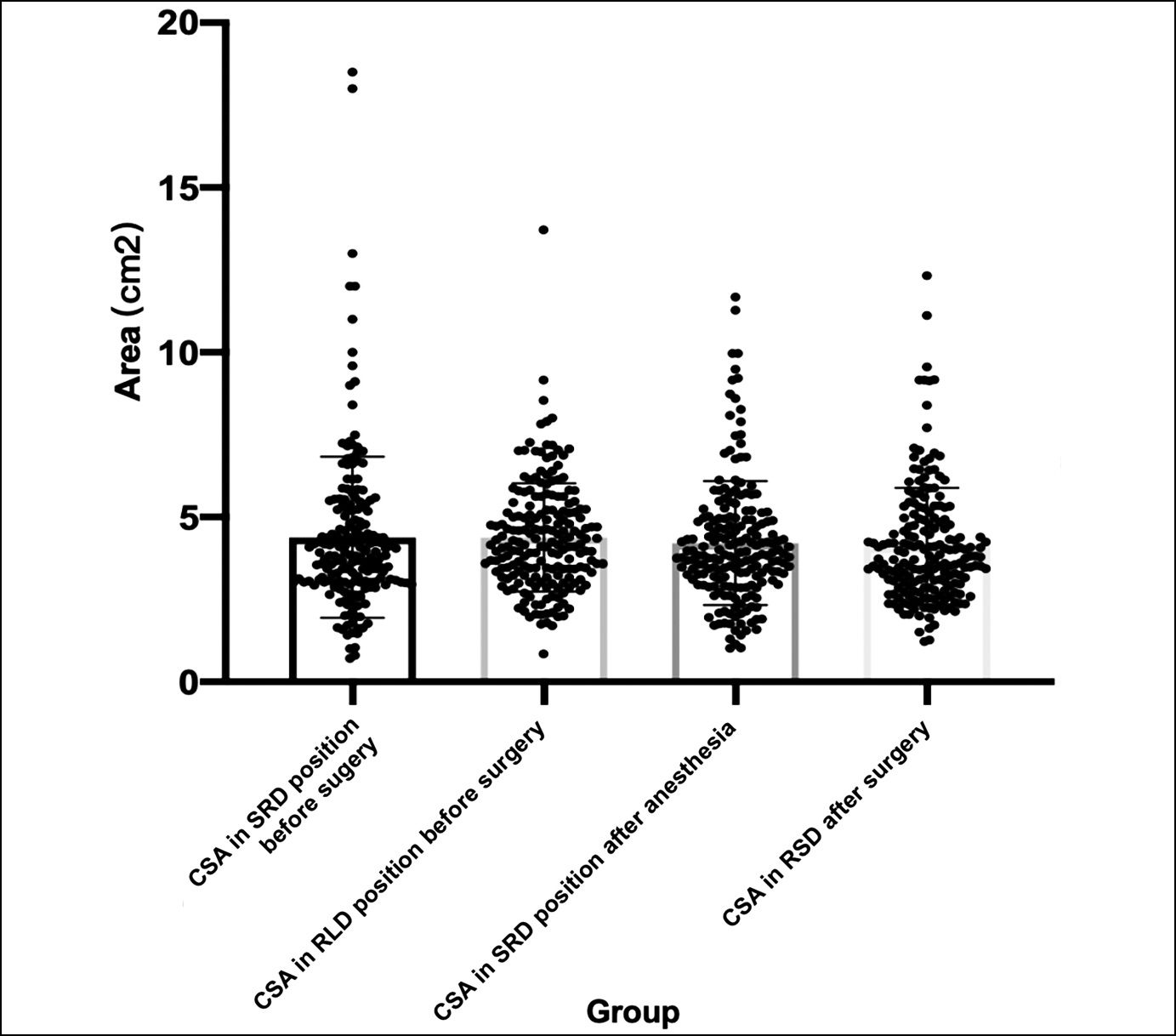 Figure 2: Multiple comparisons of CSA of gastric antrum at different time points.
Figure 2: Multiple comparisons of CSA of gastric antrum at different time points.
G-power 3.1.9.2 was used to calculate the sample size. This study was a diagnostic study based on two-classification variables, the expected sensitivity was 0.8, the expected specificity was 0.8, the sensitivity allowable error was 0.05, and the specificity allowable error was 0.05, and the test level was 0.05, considering the lost interview rate of 10%. According to the sensitivity and specificity, minimum sample size was 193.
The demographic variables were studied by descriptive analysis using computer software (SPSS, version 26.0; SPSS Inc., Chicago, IL). All data were described as mean ± standard deviation (SD) or number of cases and percentage. Shapiro-Wilk test was used to evaluate the normality of data distribution. Chi-square test was performed to compare the incidence data. Gender, history of motion sickness, history of smoking, opioid used, and gastric antral CSA in the SRD position, after anaesthesia induction were included to a binary logistic analysis. In order to evaluate the ultrasonic measurement of gastric antral CSA in predicting the occurrence and severity of PONV, receiver operating characteristic curve (ROC curve) of the subjects were drawn. The optimal cut-off value was calculated by using Youden index. The statistical significance was defined as p <0.05 (two-sided).
RESULTS
The demographic data of the patients were shown in Table I. This study showed that gender (p<0.001), age (p=0.018), history of motion sickness (p <0.001), and history of smoking (p=0.018) were significantly related to PONV, while solid fasting time (p=0.448) and liquid fasting time (p=0.083) and PONV were not statistically significant.
Table I: Demographic and clinical characteristics of the patients.
|
Variable |
PONV |
Total |
p-value* |
|
|
|
Present, n (%) |
Absent, n (%) |
|
|
|
Gender |
|
|
|
<0.001 |
|
Male |
22(11.3) |
58(29.7) |
80 |
|
|
Female |
74(37.9) |
41(21.0) |
115 |
|
|
Age, years |
|
|
|
|
|
<50 |
49(25.1) |
34(17.4) |
83 |
0.018 |
|
≥50 |
47(24.1) |
65(33.3) |
112 |
|
|
Solid fasting time |
||||
|
<10 |
11(5.6) |
15(7.7) |
26 |
0.448 |
|
≥10 |
85(43.6) |
84(43.1) |
169 |
|
|
Liquid fasting time |
|
|
|
|
|
<6 |
3(1.5) |
9(4.6) |
12 |
0.083 |
|
≥6 |
93(47.7) |
90(46.2) |
183 |
|
|
History of motion sickness |
||||
|
No |
73(37.4) |
98(50.3) |
171 |
<0.001 |
|
Yes |
23(11.8) |
1(0.5) |
24 |
|
|
History of smoking |
|
|
|
|
|
Yes |
8(4.1) |
20(10.1) |
29 |
0.018 |
|
No |
88(45.1) |
79(40.5) |
166 |
|
|
Note: *Chi-square test statistical result. |
||||
A total of 266 patients were enrolled in this study, of which 199 patients successfully measured the gastric antral CSA (74.81% of the patients). For 67 patients, gastric antral CSA could not be measured. In addition, 4 patients were excluded because of abnormal values found in the box plot. This analysis was based on the remaining 195 data sets. There was no reflux aspiration in the cohort.
The frequency of PONV in LC patients in this study was 47.69%. The gastric antral CSA in the SRD position was 438±244 mm2 before the surgery, gastric antral CSA in RLD position before the surgery was 439±164 mm2, gastric antral CSA in SRD position after anaesthesia was 421±188 mm2, and gastric antral CSA in RSD after surgery was 411±177 mm2. One-way ANOVA showed that there was no significant difference in all stages (Figure 2).
The results displayed that gastric antral CSA in SRD position after induction were significantly correlated with the incidence and severity of PONV (p<0.05). The data showed that the gastric antral CSA in SRD position after induction could be used to predict the occurrence of PONV, in which gastric antral CSA in SRD after anaesthesia induction >398.85 mm2 (AUC=0.623) highly indicated the occurrence of PONV (Figure 3), while the data of other time and posture were excluded because of relatively low accuracy.
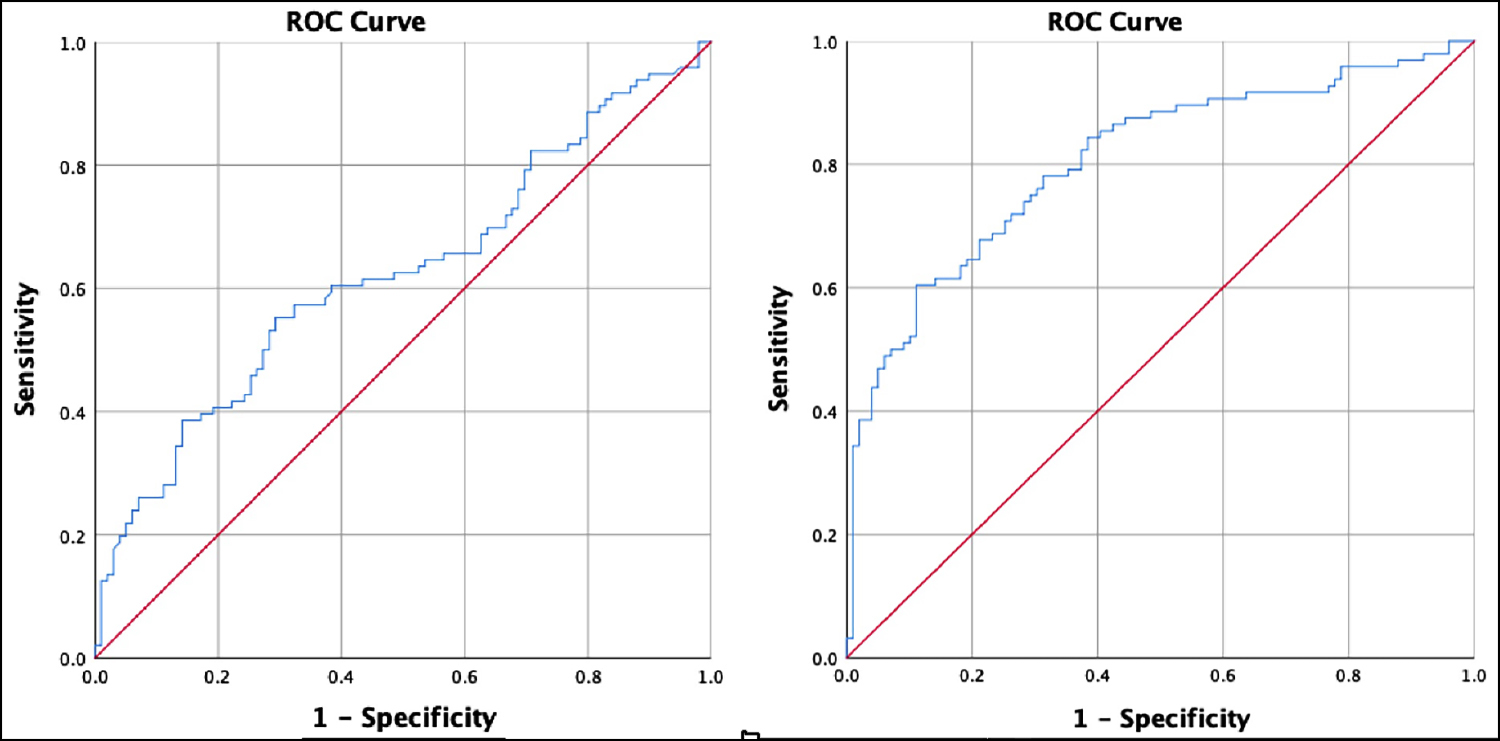 Figure 3: The ROC curve of gastric antral CSA in the SRD position after anaesthesia induction and ROC curve of subjects for predicting PONV by a binary logistics retrospective model.
Figure 3: The ROC curve of gastric antral CSA in the SRD position after anaesthesia induction and ROC curve of subjects for predicting PONV by a binary logistics retrospective model.
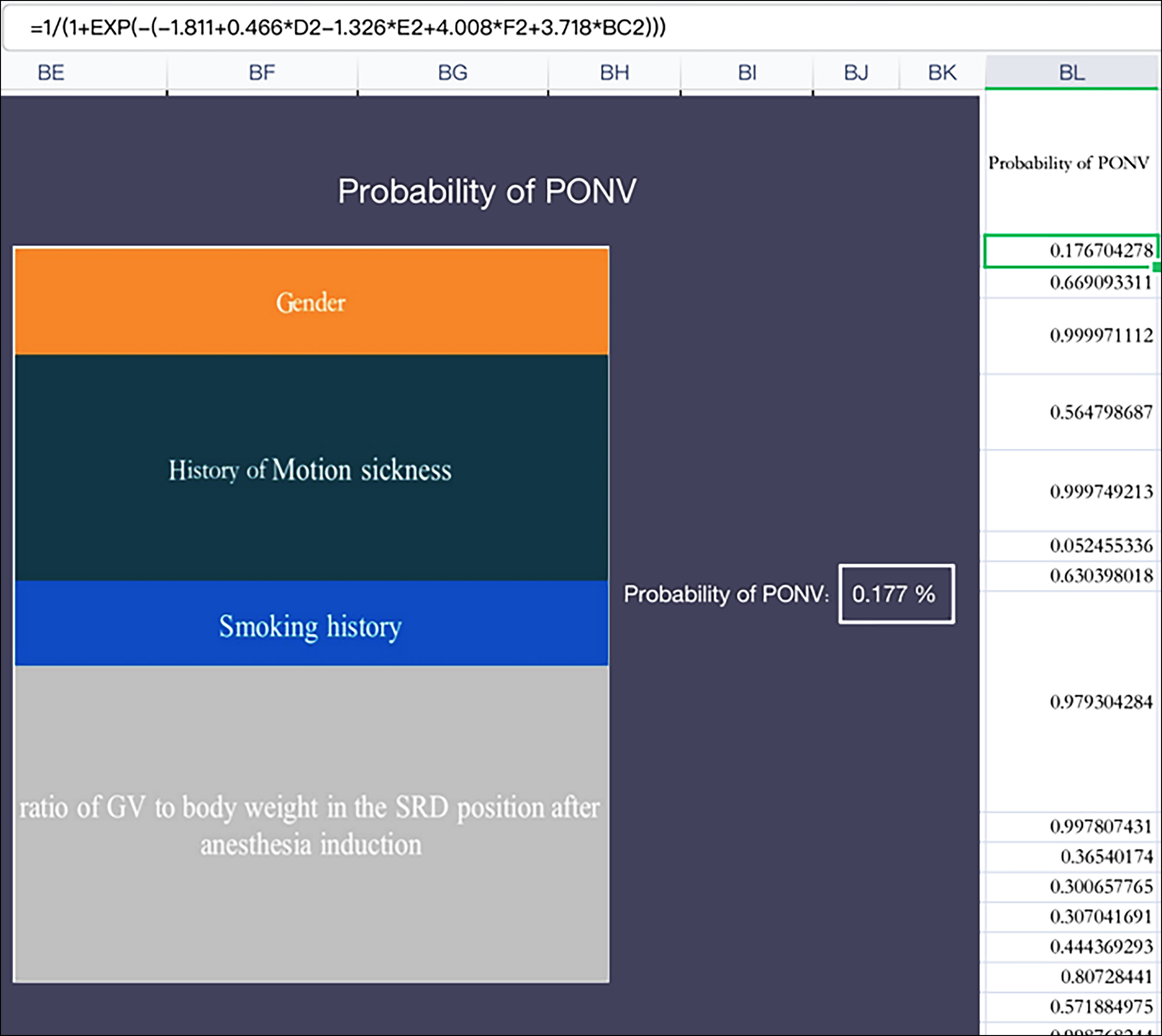 Figure 4: The risk Formula of PONV's forecasting Model.
Figure 4: The risk Formula of PONV's forecasting Model.
Gender, history of motion sickness, history of smoking, opioid dosage, and gastric antral CSA in the SRD position after anaesthesia induction were included to a binary logistic analysis. The results showed that the history of motion sickness on PONV was statistically significant (OR=49.758, 95%CI 6.068-408.34, p <0.001); the effect of smoking on PONV was statistically significant (OR=0.256, 95%CI 0.066-0.996, p=0.049); the effect of gender on PONV was statistically significant (OR=2.626, 95%CI 1.130-6.106, p=0.025); the effect of gastric antral CSA in the SRD position after anaesthesia induction on PONV was statistically significant (OR=1.517, 95%CI 1.210-1.902, p <0.001); Unexpectedly, the effect of opioid dosage on PONV was not statistically significant.
A binary logistics retrospective model consisting of history of motion sickness, history of smoking, gastric antral CSA in the SRD position after anaesthesia induction showed that the subject performance characteristic curve for predicting PONV (AUC=0.805). This was better than using gastric antral CSA in the SRD position after anaesthesia induction alone to predict PONV (Figure 3).
The risk calculation tool based on Excel was developed so that the occurrence probability of PONV could be obtained by inputting an individual's prediction data (Figure 4).
DISCUSSION
The mechanism of PONV is not determined at present. Some studies showed that the occurrence of PONV might be triggered by stimulation signals from four main functional areas, namely the cerebral cortex, the vestibular labyrinthine system, the chemoreceptor the trigger area, and the gastrointestinal vagal nervous system, in the medulla oblongata vomiting centre. It was confirmed that the receptors and neurotransmitters related to nausea and vomiting were dopamine, H1 histamine, M1 acetylcholine, opioids, 5-hydroxytryptamine (5-HT), natural killer cells (NK1), and substance P.6 Tong et al. pointed out that the risk factors for an adult PONV include female, history of PONV or history of motion sickness, using volatile anaesthetics and nitrous oxide, postoperative using of opioids, non-smokers, the duration of anaesthesia, and the type of surgery (such as cholecystectomy, laparoscopic surgery and gynaecological surgery).7
Intraperitoneal injection of CO2 during LC surgery would increase intraabdominal pressure, which would have a significant effect on mechanical ventilation, and reduce diaphragm deviation and pulmonary compliance. This can lead to an increase in PAP and a decrease in end-expiratory tidal volume, which might lead to CO2 retention and acidosis,8 this might increase the incidence of PONV in patients. Although previous studies showed that ultrasonic measurement of gastric antral CSA could reflect GV and had certain clinical value in predicting nausea and vomiting, and that the incidence of vomiting increased significantly with the increase of GV per unit body weight, other studies showed that the increase of GV was a marker of perioperative pulmonary aspiration and reflux and the risk of PONV.4 However, no study evaluated the gastric antral CSA threshold that might predict PONV. This study further confirmed the association between gastric antral CSA in SRD after anaesthesia induction.
In addition, in this study, the mathematical calculation of the gastric antral CSA and the qualitative analysis of liquid contents were needed to flip patients to RLD, and because all patients in this study were routine surgical patients, they needed to fast before surgery, which was not feasible in emergency surgery patients, so this forecast could not be used to predict the incidence and severity of PONV in patients undergoing emergency surgery. In addition, a risk calculation tool based on Excel was developed in this study, so that the occurrence probability of PONV could be obtained by inputting an individual's prediction data. This could help the authors to quickly and accurately screen high-risk patients in PONV.
It was well known that signals from the intestinal tract activate the vagus nerve through the intestinal sensory system.9 As an established biomarker of nausea and vomiting, gastric dysrhythmia could stimulate the vagus nerve after stimulating local intestinal neurons. Therefore, after some surgeries that might induce PONV, such as laparoscopic surgery, common side effects such as postoperative intestinal obstruction and subsequent interruption of gastrointestinal motility rhythm might be the cause of PONV.10 This explained why gastric antral CSA could be used to predict the occurrence of PONV.
This study showed that the incidence of PONV in females was higher than that in males, which was consistent with the conclusion of Jaesoon et al.11 Gender could affect the incidence of PONV in patients, and females were a high risk factor for PONV. In addition, the average gastric antral CSA of female before anaesthesia was larger than that of male. This suggested that when predicting PONV for ultrasonic measurement of gastric antral CSA, different boundary values of gastric antral CSA should be used to predict PONV, according to the difference between men and women. Studies showed that women have slower gastric emptying rates, especially solids and liquids, than men.12 The gender difference in gastrointestinal motility might be caused by female sex hormones. Compared with premenopausal and postmenopausal women who did not receive hormone replacement therapy, premenopausal and postmenopausal women who received hormone replacement therapy had slower gastric emptying.13 Some experiments showed that the women's postprandial symptoms last longer, which might be related to delayed gastric emptying, while patients with delayed gastric emptying were related to women, postprandial satiety and nausea.14
This study showed that age (p=0.018) was significantly correlated with the incidence and severity of PONV, and the incidence and severity of PONV decreased with the increase of age. However, all the people included in this study are adults, and the underlying mechanism might be due to the decrease of the autonomic nerve reflex with the increase of age. But there was no statistical significance between preoperative solid/liquid fasting time and PONV, which might be due to the long solid/liquid fasting time of patients.
A meta-analysis showed that there is a strong evidence that anaesthesia containing opioids does not reduce postoperative pain but leads to more PONV than anaesthesia without opioids.15 Apfel et al. found that fentanyl, alfentanil, and sufentanil had little effect on PONV compared with volatile anaesthetics in otorhinolaryngology and strabismus surgery,16 Smith et al. when fentanyl was used for anaesthesia induction, the absolute risk of PONV was reduced by 14%.17 It might be due to the fear of delayed awakening, respiratory depression and other adverse reactions, that anaesthesiologists were reluctant to use sufficient opioids during surgical surgery.18
This research had some limitations. First of all, it was just a single-centre trial. Secondly, it was performed on adults undergoing LC surgery. The results and numerical models should not be extrapolated to other subjects such as diabetes, morbidly obese, and the patients with specific symptoms. Finally, the inherent limitations of ultrasound also applied, including the need for an appropriate soft tissue window to obtain an image.
CONCLUSION
Gastric US assessment of gastric antral CSA might change the current assessment model of PONV risk. This study showed that gastric antral CSA in the SRD position after anaesthesia induction and a binary logistics retrospective model could be used to predict the occurrence of PONV, which could be helpful to adjust intraoperative and postoperative interventions and accelerate the recovery of patients.
ETHICAL APPROVAL:
This study was performed with the approval of the Ethical Committee in the First Affiliated Hospital of Wannan Medical College (Approval No. 2022/02).
PATIENTS’ CONSENT:
Informed consent was obtained from all patients and their relatives before the study began.
COMPETING INTEREST:
The authors declared no potential competing interest with respect to the research, authorship, and publication of this study.
AUTHORS’ CONTRIBUTION:
KW: Drafting the manuscript, acquisition, analysis, and interpretation of data.
JT: Supervision, review writing, and editing.
ZH: Critical revision of important work content.
ZG: Data curation, supervision, review writing, and editing.
JL: Formal analysis, investigation, and resources.
WG: Drafting, conception, designing the study content, review writing, and editing.
All authors have read and approved the final version of the manuscript to be published.
REFERENCES
- Massoth C, Schwellenbach J, Saadat-Gilani K, Weiss R, Pöpping D, Küllmar M, et al. Impact of opioid-free anaesthesia on postoperative nausea, vomiting and pain after gynaecological laparoscopy - A randomised controlled trial. J Clin Anesth 2021; 75:110437. doi: 10.1016/j.jclinane.
- Valero Castañer H, Vendrell Jordà M, Sala Blanch X, Valero R. Preoperative bedside ultrasound assessment of gastric volume and evaluation of predisposing factors for delayed gastric emptying: a case-control observational study. J Clin Monit Comput 2021; 35(3):483-9. doi: 10.1007/s10877- 020-00489-9.
- Bouvet L, Zieleskiewicz L, Loubradou E, Alain A, Morel J, Argaud L, et al. Reliability of gastric suctioning compared with ultrasound assessment of residual gastric volume: A prospective multicentre cohort study. Anaesthesia 2020; 75(3):323-30. doi: 10.1111/anae.14915.
- Temel ME, Totoz T, Erkalp K, Temel GS, Selcan A. A randomised, double-blind study of the ultrasound assessment of the effect of pharyngeal packing on peri-operative gastric volume in nasal surgery. BMC Anesthesiol 2019; 19(1):121. doi: 10.1186/s12871-019- 0786-7.
- Van de Putte P, Perlas A. Ultrasound assessment of gastric content and volume. Br J Anaesth 2014; 113(1):12-22. doi: 10.1093/bja/aeu151.
- Gan TJ. Mechanisms underlying postoperative nausea and vomiting and neurotransmitter receptor antagonist-based pharmacotherapy. CNS Drugs 2007; 21(10):813-33. doi: 10.2165/00023210-200721100-00003.
- Gan TJ, Belani KG, Bergese S, Chung F, Diemunsch P, Habib AS, et al. Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg 2020; 131(2):411-48. doi: 10.1213/ANE.0000000000004833.
- Son JS, Oh JY, Ko S. Effects of hypercapnia on postoperative nausea and vomiting after laparoscopic surgery: A double-blind randomized controlled study. Surg Endosc 2017; 31(11):4576-82. doi: 10.1007/s00464-017-5519-8.
- Percie du Sert N, Chu KM, Wai MK, Rudd JA, Andrews PL. Telemetry in a motion-sickness model implicates the abdominal vagus in motion-induced gastric dysrhythmia. Exp Physiol 2010; 95(7):768-73. doi: 10.1113/expphysiol. 2009.052001.
- Kimura H, Yamazaki T, Mihara T, Kaji N, Kishi K, Hori M. Purinergic P2X7 receptor antagonist ameliorates intestinal inflammation in postoperative ileus. J Vet Med Sci 2022; 84(4):610-617. doi: 10.1292/jvms.22-0014.
- Son J, Yoon H. Factors Affecting postoperative nausea and vomiting in surgical patients. J Perianesth Nurs 2018; 33(4):461-470. doi: 10.1016/j.jopan.2016.02.012.
- Mori H, Suzuki H, Matsuzaki J, Taniguchi K, Shimizu T, Yamane T, et al. Gender difference of gastric emptying in healthy volunteers and patients with functional dyspepsia. Digestion 2017; 95(1):72-78. doi: 10.1159/000452359.
- Hutson WR, Roehrkasse RL, Wald A. Influence of gender and menopause on gastric emptying and motility. Gastroenterology 1989; 96(1):11-7. doi: 10.1016/0016- 5085(89)90758-0.
- Mearadji B, Penning C, Vu MK, van der Schaar PJ, van Petersen AS, Kamerling IM, et al. Influence of gender on proximal gastric motor and sensory function. Am J Gastroenterol 2001; 96(7):2066-73. doi: 10.1111/j.1572- 0241.2001.03940.x.
- Frauenknecht J, Kirkham KR, Jacot-Guillarmod A, Albrecht E. Analgesic impact of intra-operative opioids vs. opioid-free anaesthesia: A systematic review and meta-analysis. Anaesthesia. 2019; 74(5):651-62. doi: 10.1111/anae. 14582.
- Apfel CC, Kranke P, Katz MH, Goepfert C, Papenfuss T, Rauch S, et al. Volatile anaesthetics may be the main cause of early but not delayed postoperative vomiting: A randomized controlled trial of factorial design. Br J Anaesth 2002; 88(5):659-68. doi: 10.1093/bja/88.5.659.
- Smith I, Walley G, Bridgman S. Omitting fentanyl reduces nausea and vomiting, without increasing pain, after sevoflurane for day surgery. Eur J Anaesthesiol 2008; 25(10): 790-9. doi: 10.1017/S026502150800464X.
- Wheeler M, Oderda GM, Ashburn MA, Lipman AG. Adverse events associated with postoperative opioid analgesia: A systematic review. J Pain 2002; 3(3):159-80. doi: 10. 1054/ jpai.2002.123652.