Association between Chronic Liver Disease Caused by Viral Hepatitis, Hospitalisation for COVID-19 and Mortality
By Javaria Aslam1, Fahad Qaisar2, Mutahra Khaliq2, Maryam Khalid3, Dur-e- Sabeeh1, Asfand Yar Ali2Affiliations
doi: 10.29271/jcpsp.2023.06.647ABSTRACT
Objective: To evaluate the association between chronic liver disease (CLD) caused by viral hepatitis and COVID-19 hospitalisation, as well as the risk of disease progression and mortality among COVID-19 hospitalised patients in relation to their prior CLD status.
Study Design: A cohort study.
Place and Duration of the study: Bahawal Victoria Hospital and Sir Sadiq Abbasi hospital, affiliated with Qauid-e-Azam Medical College, Bahawalpur, Pakistan, from July to December 2021.
Methodology: In the main group analysis, the risk of hospitalisation for COVID-19 among CLD patients was determined, with the presence of CLD due to chronic viral hepatitis B and C as the exposure variable and hospitalisation for COVID-19 as the outcome measure. Patients hospitalised for a medical condition other than COVID-19 (non-COVID medical admissions) served as an external control group. In the sub-group analysis, the risk of disease severity and mortality were determined among COVID-19 admitted patients having a prior status of CLD, with disease progression to death serving as the primary outcome measure while the exposure variable remained the same as in the main analysis.
Results: A total of 3,976 participants [mean age 51 ±14.8 years; 54.1% men; 1616 hospitalised with COVID-19, including 27 (1.7%) exposed to CLD; and 2,360 non-COVID medical admissions, including 208 (8.8%) exposed to CLD] were evaluated. There was less likelihood of hospitalisation for COVID-19 among patients with CLD (1.7% vs. 8.8%; RR=0.270; 95% CI=0.189, 0.386; p<0.001). There was less risk of death among CLD patients admitted for COVID-19 when compared with those admitted for non-COVID CLD-related complications (14.8% vs. 35.1%; RR= 0.422; 95% CI=0.168-1.06; p=0.035). Among COVID-19 admissions, CLD was associated with a decreased risk of death compared with other comorbid conditions (14.8% vs. 36.9%; RR=0.401; 95% CI=0.162-0.994; p=0.04).
Conclusion: CLD caused by viral hepatitis was significantly less likely to be present among COVID-19 hospitalised patients. There was a lower risk of severe COVID-19 and mortality owing to it among CLD patients compared to those with other comorbid conditions.
Key Words: COVID-19, Hospitalisations, Chronic liver disease, Viral hepatitis, COVID-19 severity, Death outcome.
INTRODUCTION
The COVID-19 pandemic is delivering ongoing damage from new variants and sub-variants, albeit with diminished severity due to the SARS-CoV-2 vaccine.1,2 As of August 16, 2022, more than 588 million confirmed cases and 6.4 million deaths have been reported worldwide.3 COVID-19 has been found to be worse in people with diabetes, heart disease, obesity, pulmonary disease, immunosuppressive therapy, transplant recipients, chronic kidney disease, and non-alcoholic and alcoholic fatty liver disease, which cause chronic liver disease (CLD).4
CLD, caused by viral hepatitis, alcohol-associated liver disease, and metabolism-associated liver disease (NAFLD), was one of the leading causes of mortality in 2017, accounting for 2.2% of all deaths globally.5 Numerous studies have collected data demonstrating the severity of COVID-19 in patients with CLD caused by MAFLD and alcoholic liver disease, which are independent risk factors for having severe COVID-19 themselves, whereas the relationship between COVID-19 and CLD caused by viral hepatitis, such as HCV and HBV, has received very less attention.6-8 Around 64 to 103 million people worldwide are chronically infected with Hepatitis C virus (HCV), and chronic Hepatitis B Virus infection together with HCV infection accounts for 63% of the global burden of CLD.9,10 The World Health Organisation (WHO) has already established global eradication targets for HCV by 2030.11 In Pakistan, where a national survey was conducted in 2007–2008, the prevalence of HCV was estimated to be 4.8%, ranking it second in the world.12 The incidence of viral hepatitis in patients with chronic liver disease-related sequelae accounts for a substantial share of the inpatient and outpatient burden in Pakistan.13,14
Therefore, it is plausible that patients with CLD would have been exposed to SARS-CoV-2 during the pandemic. In addition, CLD patients are frequently expected to visit hospitals, making them prone to SARS-CoV-2 infection. Furthermore, the SARS-CoV-2 infection causes lymphopenia, and deranged (FDP’s) fibrinogen degradation products,15 which already exist in CLD patients due to suppression of bone marrow and cirrhosis-associated immune dysfunction syndrome (CAIDS).16
The rationale of this study was to understand the risk of hospitalisation for COVID-19 among patients with CLD due to viral hepatitis, as well as the severity of COVID-19 and mortality among these patients. So that these individuals can receive hospital care and be vaccinated against SARS-COV-2 on a high-priority basis. The objective of the study was to evaluate the association between CLD caused by viral hepatitis and COVID-19 hospitalisation, as well as the risk of disease progression and mortality among COVID-19 hospitalised patients in relation to their prior CLD status.
METHODOLOGY
Two hospitals, Bahawal Victoria Hospital and Sir Sadiq Abbasi Hospital, a COVID-designated hospital, affiliated with the Qauid-e-Azam Medical College in Bahawalpur, Pakistan, participated in this research. After approval by the ethical review board, vide number 1072/DME/QAMC Bahawalpur, a retrospective cohort study was conducted from 1st July to 31st December 2021. The STROBE reporting standards were followed for this study.
The association between hospitalisation for COVID-19 and previous CLD was evaluated using a retrospective cohort design. In this investigation, CLD is taken as an exposure and hospitalisation for COVID-19 is taken as an outcome measure, using the non-COVID medical admissions as external control group against COVID-19 hospitalisations. They were COVID-19 syndrome-negative medical admissions who tested negative for SARS-CoV-2 by Real Time-PCR. In the subgroup analysis that only looked at COVID-19-related hospitalisations, the severity of COVID-19 and death rates were compared between patients with chronic liver disease and those without it. In this analysis, disease progression to death was taken as the primary outcome and disease severity, presence or absence of hypoxemia at the time of admission, need for ICU care and hospital stay among discharged patients as secondary outcome measures, keeping the same exposure variable as in the main group analysis.
Through a daily assessment of hospital admissions, study sites assessed the possible eligibility of hospitalised persons aged 17 and above. COVID-19 cases were patients hospitalised within 10 days of symptom onset with clinical symptoms of acute COVID-19 and a positive PCR for SARS-CoV-2 or suggestive HRCT chest for COVID pneumonia. Sites aimed to identify every case admitted to the hospital during the study duration. Approximately 1:1 ratio of non-COVID patients were recruited from eligible medical admissions within two weeks after COVID-19 case recruitment to eliminate lead time bias. After enrolling patients, information on chronic liver disease was obtained. Skilled personnel collected demographic, clinical, radiological and laboratory data through medical record checks. Testing for SARS-CoV-2 was done using reverse transcriptase–PCR (polymerase chain reaction) on specimens taken from the upper respiratory tract by trained personnel. Ultrasound for liver texture and HRCT chest was reported by a Consultant Radiologist.
CLD or liver cirrhosis was diagnosed by the radiological presence of bright coarse echo texture of the liver and its nodular surface with or without portal hypertension, on Doppler ultrasound done during the same admission or within six months of the most recent admission. Patients with causes of liver cirrhosis other than viral hepatitis HCV or HBV were excluded from both the COVID and non-COVID admissions. Those who had hepatocellular carcinoma (HCC), metastatic disease or other malignant focal liver masses were also excluded. The Child-Pugh-Turcotte score was used to determine the severity of liver disease, with A representing normal liver function, B representing moderately decreased hepatic function, and C representing severe hepatic dysfunction.17 MELD scores of 10, 10 to 19, and >19 correspond to CTP classes A, B, and C, respectively.18
Data on the severity of COVID-19 admissions were collected. The outcome information was collected within 28 days of hospital admission or until hospital discharge, whichever happened first. The primary outcome was a binary measure that distinguished between patients who died and those who did not. As a secondary assessment, the authors categorised COVID-19 severity using World Health Organization COVID-19 Clinical Progression Scale that ranges from uninfected (level 0) to death (level 9). According to the scale, level 4 indicated a moderate disease (without hypoxemia and no need for oxygen), level 5 a severe disease (hypoxemic and needed oxygen support via nasal prongs or non-re-breather (mask), and levels 6, 7, and 8 as critical illness, (on high flow oxygen support via High flow nasal cannula HFNC or noninvasive ventilator support or required organ support or renal replacement therapy). Levels 6, 7, 8, and 9 necessitated intensive care unit (ICU) admission. The authors also categorised the severity of COVID-19 based on the length of hospitalisation while taking the chance of death into account.
Risk of having CLD (exposed) versus not having CLD (unexposed) was compared between patients hospitalised for COVID-19 and patients hospitalised for other medical conditions in order to determine the association between hospitalisation for COVID-19 and prior CLD status. Chi-square test was conducted to calculate the relative risk (RR) with 95% CI. In this model, hospitalisation due to COVID-19 was associated with lower chances of having CLD if the RR was less than 1.0. Risk estimation was calculated as = (1- OR)*100. COVID-19 hospitalised patients and non-COVID medical admissions were analysed to evaluate the frequency and proportion of CLD patients with reference to its cause. In addition to determining the frequency and percentage of medical admission types among non-COVID admitted patients, the frequency and proportion of comorbid conditions among COVID-19 hospitalisations were also determined. Child scores were calculated for CLD patients among COVID-19 as well as non-COVID medical admissions, and associated complications or recent decompensation of portal hypertension with its manifestation were also noted. The death probability of COVID-19 patients with CLD was compared with those of non-COVID CLD patients, taking their child scores into account.
In a subgroup analysis, the risk of disease progression to death among COVID-19 admissions was assessed in relation to exposure to CLD. Patients with CLD were compared to those with other risk factors (diabetes, cardiovascular disease, old age, chronic kidney disease, pregnancy, pulmonary disease, and immunosuppressive medicines) or no risk factors for the severity of COVID-19 and disease progression to death. For this, cross tabulation and Chi-square test were done to calculate RR and post hoc sensitivity analysis was done to calculate p-value for the multiple comparisons. An RR less than 1.0 suggested a decreased likelihood of CLD patients experiencing higher COVID-19 severity levels when compared to non-CLD patients. The probability of discharge from the hospital was assessed within 28 days following admission in CLD vs. non-CLD patients. Cumulative incidence function curves were created for hospital stays in days, with hospital discharge as the primary event, for patients with Diabetes, cardiovascular disease, and chronic liver disease. Version 26 of IBM SPSS was used for statistical analysis. Statistical significance was indicated by 95% confidence intervals excluding the null or a 2-sided p <0.05.
RESULTS
A total of 4,799 patients were enrolled from July 1st, 2021 to December 31st, 2021 at both hospitals; 823 patients were excluded for a number of reasons, with the most common being an undetermined PCR status (n=520) or unidentified cause of medical admission (n=271) or an unknown cause of chronic liver disease (n=32), among both COVID-19 admissions (n=3) and non-COVID admissions (n=29, Figure 1).
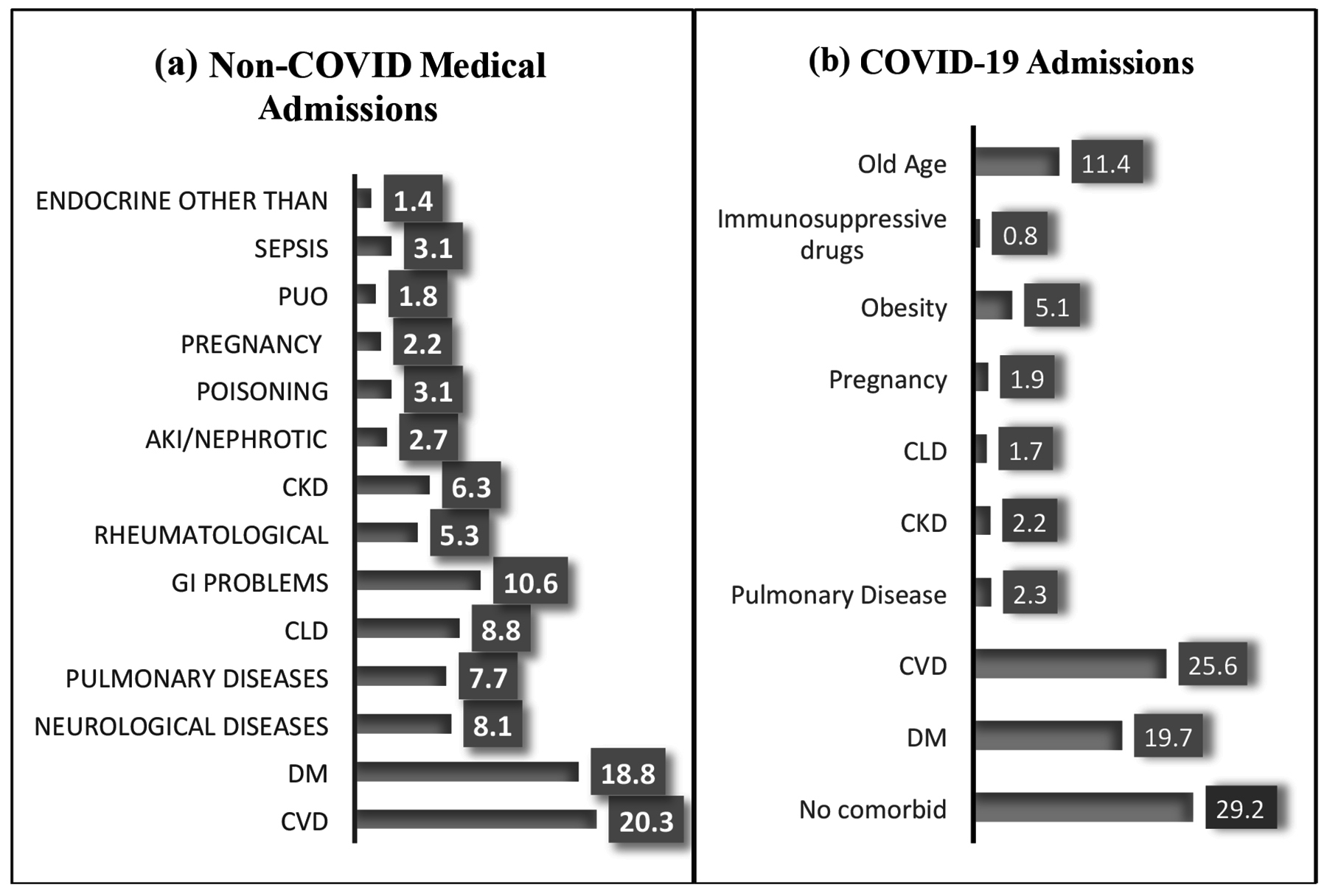 Figure 1: (a) Illustrates the percentage of non-COVID medical admissions based on the diagnosis. (b) Shows the percentage of COVID-19 admissions based on pre-existing comorbid conditions.
Figure 1: (a) Illustrates the percentage of non-COVID medical admissions based on the diagnosis. (b) Shows the percentage of COVID-19 admissions based on pre-existing comorbid conditions.
DM = Diabetes mellitus, CVD = Cardiovascular disease, PUO = Pyrexia of unknown origin, AKI = Acute kidney injury), CKD = Chronic kidney disease, GI = Gastrointestinal problems, pregnancy related medical complications, CLD = Chronic liver disease.
The total analytic population included 3,976 participants [mean age, 51 ±14.8 years; 2173 (54.1%) men; 1616 (40.6%) patients hospitalised with COVID-19, including 27 patients exposed to CLD; 2360 (59.4%) non-COVID medical admissions, including 208 patients exposed to CLD]. Characteristics of both groups are given in Table I.
The percentages of non-COVID hospitalisations by their diagnosis and the percentage of COVID-19 hospitalisations by their comorbid diseases are depicted in Figure 1.
Two hundred and eight (8.8%) non-COVID medical admissions vs. 27(1.7%) COVID-19 admissions, had viral hepatitis induced CLD. So, there was a decreased probability of CLD among COVID-19 hospitalisations, [Absolute difference, 7.1%; 95% CI = (5.8-8.4); RR = 0.270; 95% CI = (0.189, 0.386); p<0.001). CLD was associated with a decreased risk of death among COVID-19 hospitalisations compared to those admitted with other CLD-related complications without COVID-19, [4(14.8%) vs. 73(35.1%); Absolute difference, 20.3%; 95% CI = (1.59%-31.44%); RR = 0.422; 95% CI = (0.168, 1.06); p = 0.06). Among the deceased, three (75%) COVID-19 patients with CLD, had upper Gastrointestinal (GI) bleed and one (25%) had acute on chronic jaundice, whereas among non-COVID-19 CLD admissions, four (5.5%) died from spontaneous bacterial peritonitis, twenty-four (32.9%) from hepatic encephalopathy, twenty-four (32.9%) from upper GI bleed, fifteen (20.5%) from Hepato-renal syndrome, and six (8.2%) from acute on chronic jaundice. Comparing the death outcome among both groups, in relation to their Child Pugh Score, there was no significant association between death outcome with child score A, B and C, [0% case vs. 0% control; RR, 2.29; 95% CI = (0.047, 110.91); p = 0.67], [1(14.3%) vs. 3(6.8%); RR, 2.44; 95% CI, (0.300, 19.88); p = 0.403] and [3(60%) vs. 70(55.6%); RR, 1.08; 95% CI, (0.519, 2.24); p = 0.83], respectively.
In the subgroup analysis, there were total 1616 COVID-19 related hospitalisations, [mean age 52 yrs. ±17, 55.9% males, the mean number of comorbid conditions 1.2±1 minimum 0, maximum 5], patients with CLD were compared with those who had other risk factors or no risk factors with regard to the severity of the illness and the risk of mortality. CLD among COVID-19 patients was associated with decreased risk of death when compared to those who did not have CLD, [Four (14.8%) patients with CLD vs. 586 (36.9%), absolute difference 22.1%; 95% CI, (4.28% to 31.31%); RR, 0.401; 95% CI, (0.162, 0.994); p=0.04]. COVID-19 patients with CLD were less likely to have admission hypoxemia, [13 (48.1%) vs. 1275 (80.23%), Absolute difference 32.1%; 95% CI, (14.1% to 49.6%); RR, 0.600; 95% CI, (0.405, 0.888); p=0.01] and, they were less likely to receive ICU care, [Three (11.1%) vs. 727 (45.8%), Absolute difference 34.7%; 95% CI, (17.5% to 42.3%); RR, 0.242; 95% CI, (0.083, 0.707); p<01]. Using cross-tabulation and a post hoc sensitivity test, the outcomes of patients with CLD and those with other comorbid conditions were compared separately.
Table I: The demographic characteristics, cause, and severity of CLD in COVID-19 and non-COVID hospitalisations.
|
Characteristics |
COVID-19 Hospitalisations n (%) |
Non-COVID Hospitalisations n (%) |
||
|
CLD (Exposed) |
Non-CLD (Unexposed) |
CLD (Exposed) |
Non CLD (Unexposed) |
|
|
Age years Mean±SD |
52 ±17 |
51±13 |
||
|
16-45 |
5(18.5) |
536(33.7) |
58(27.9) |
645(30) |
|
46-65 |
17(63) |
702(44.2) |
121(58.2) |
1249(58) |
|
66-80 |
5(18.5) |
279(17.6) |
25(12) |
251(11.7) |
|
≥81 |
0(0) |
72(4.5) |
4(1.9) |
7(0.3) |
|
Gender |
|
|
|
|
|
Male |
14(51.9) |
890(56) |
106(51) |
1163(54) |
|
Female |
13(48.1) |
699(44) |
102(49) |
989(46) |
|
Cause of CLD |
|
|
|
|
|
Hep C |
24(88.9) |
0 |
168(80.8) |
0 |
|
Hep B |
3(11.1) |
0 |
31(14.9) |
0 |
|
Hep B +C |
0 |
0 |
9(4.3) |
0 |
|
Child Pugh Score |
|
|
|
|
|
“A” |
15(55.6%) |
0 |
38(18.2) |
0 |
|
“B” |
7(25.9%) |
0 |
44(21.2) |
0 |
|
“C” |
5(18.5%) |
0 |
126(60.6) |
0 |
|
Complications related to CLD |
|
|
|
|
|
No complication |
19(69.3) |
0 |
0 |
0 |
|
Upper GI bleed |
3(11.5) |
0 |
61(29.3) |
0 |
|
Acute on chronic hepatitis |
4(15.4) |
0 |
11(5.3) |
0 |
|
Hepatic Encephalopathy |
1(3.8) |
0 |
62(29.8) |
0 |
|
SBP |
0 |
0 |
24(11.5) |
0 |
|
HRS |
0 |
0 |
22(10.6) |
0 |
|
Refractory Ascities |
0 |
0 |
28(13.5) |
0 |
|
Standard deviation (SD), Chronic liver disease (CLD), Upper gastro intestinal bleed (UGIB), Spontaneous bacterial peritonitis (SBP), Hepatorenal syndrome (HRS). |
||||
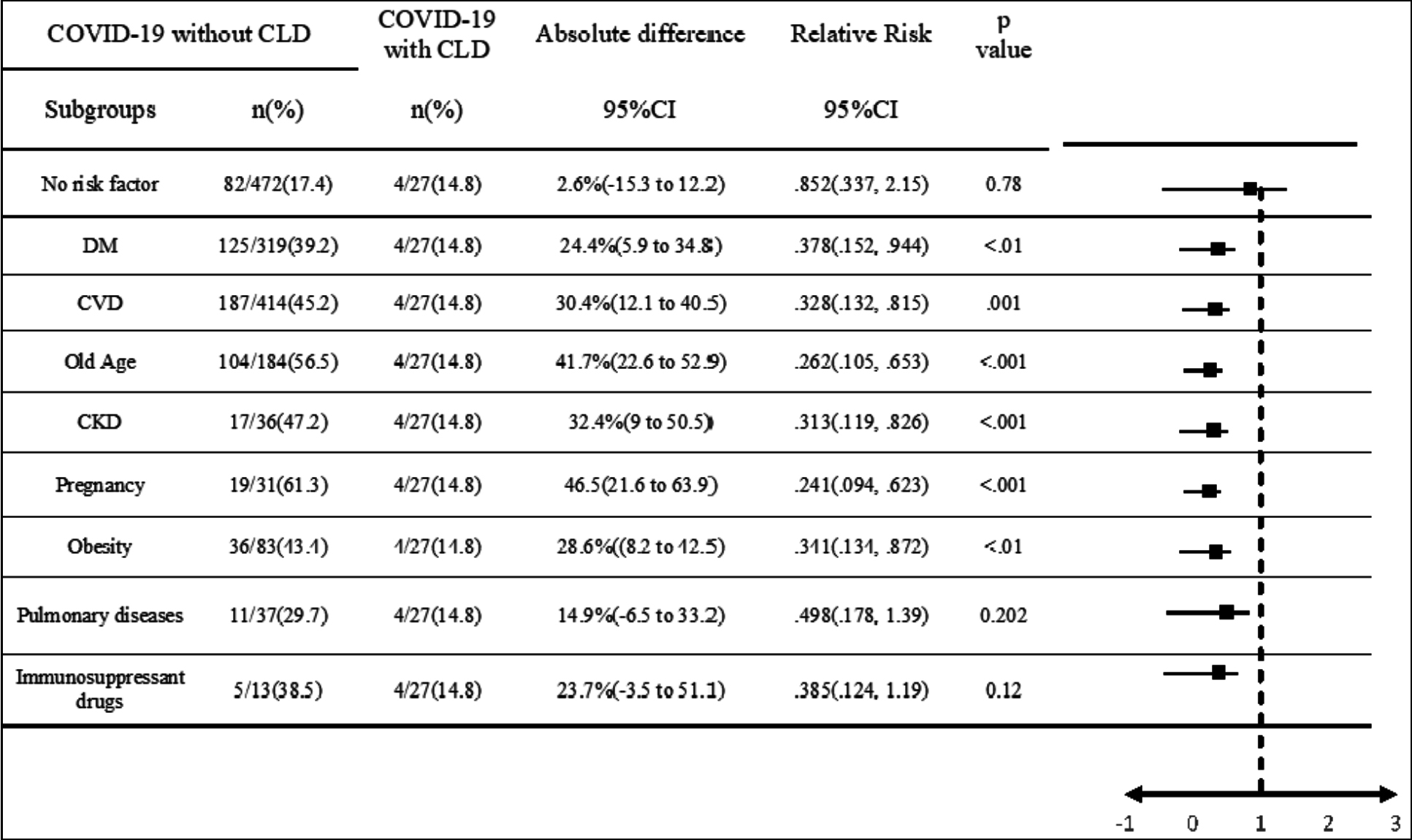 Figure 2: Statistical comparison of mortality among COVID-19 admissions with CLD and other comorbid comorbidities.
Figure 2: Statistical comparison of mortality among COVID-19 admissions with CLD and other comorbid comorbidities.
CLD = Chronic liver disease, DM = Diabetes mellitus, CVD = Cardiovascular disease, CKD = Chronic kidney disease. It compares the relative risk (RR) of mortality among COVID-19-admitted patients with and without CLD, divided into subgroups based on their co-morbid conditions. RR is calculated via cross-tabulation and Chi-square test and p-value was calculated with post-Hoc sensitivity test. The risk estimation formula is (1-RR) * 100. The effect sizes are displayed in a forest plot, and deviations from the null are deemed insignificant given the p-value.
COVID-19 patients with CLD had a significantly lower risk of death when compared to diabetes mellitus, cardiovascular diseases, old age, chronic kidney disease, pregnancy, and obesity as comorbid conditions, however, there was no significant difference between patients with no risk factors, pulmonary diseases, and use of immunosuppressant treatments, as shown in Figure 2.
When pulmonary disease severity was compared among the COVID-19 patients with different comorbid conditions, patients with CLD were more likely to be admitted with moderate disease than those with diabetes mellitus [15 (55.6%) vs. 29 (9.1%); RR, 6.11; 95% CI, (3.76, 9.91); p<0.001], cardiovascular disease [15 (55.6%) vs. 22 (5.3%); RR, 10.45; 95% CI, (6.16, 17.73); p<0.001], and chronic kidney disease [15 (55.6%) vs. 2 (5.6%); RR, 10.55; 95% CI, (2.62, 42.40), p<0.001]. Among COVID-19 hospitalised patients who improved and were discharged from the hospital, those with CLD had an insignificantly shorter hospital stay (1-5 days) when compared to those without any risk factor [21(91.3%) vs. 374(79.2%); RR, .981; 95%CI, (0.7982 to 1.20); p=.86], with diabetes mellitus [21(91.3%) vs. 210(65.8%); RR, 1.18; 95%CI, (0.951, 1.46); P=0.13], and with cardiovascular disease [21(91.3%) vs. 260(62.8%); OR, 1.23; 95%CI, (0.99, 1.53); p=.05].
DISCUSSION
In this multicentre retrospective cohort study, chronic liver disease due to viral hepatitis B and C was found significantly lower among COVID-19 related hospitalisations as compared to non-COVID medical admissions. Chronic liver disease among COVID-19 patients was associated with decreased severity of pulmonary disease and decreased risk of death when compared to those with other risk factors like diabetes mellitus, cardiovascular diseases, old age, chronic kidney disease, pregnancy, and obesity. However, the risk of death did not differ significantly between patients with chronic liver disease and those with no risk factor, pulmonary disease, or on immunosuppressant medicines.
When compared to those who did not have chronic liver disease, COVID-19 patients with chronic liver disease were less likely to have admission hypoxemia and the need for shifting to the ICU for invasive or non-invasive respiratory support. COVID-19 patients with chronic liver disease were more likely to be admitted with moderate pulmonary disease than those with diabetes mellitus, cardiovascular disease, or CKD. Changes in innate and adaptive immunity among individuals with liver cirrhosis may account for a lower dysregulated immune response to SARS-CoV-2, resulting in a reduction in the severity of pulmonary illness.19 In this way, the study gives a unique comparison of COVID-19 severity among patients with different comorbid conditions.
In a systematic review and meta-analysis based on 73 studies, the prevalence of CLD among COVID-19 patients was shown to be only 3%. In both COVID-19 positive and negative hospitalisations, the prevalence of CLD was the same.6 Roughly the same frequency of CLD among COVID-19-related hospitalisations (1.7%) was observed in this study, although non-COVID CLD-related medical admissions were greater in comparison (8.8%), which can be explained by the fact that Pakistan has the highest prevalence of both HCV and CLD.12,13
About 3.8% of hospitalised CLD patients with COVID-19 had hepatic encephalopathy, 11.5% had upper GI bleeding, and 15.4% had acute on-chronic hepatitis. Three (75%) of the deceased cases had upper GI bleed and one (25%) had acute on chronic hepatitis, compared to 32.9% of the controls who died from upper GI bleed and 8.2% from acute on-chronic hepatitis.19,20 Comparing the death outcome of CLD patients with and without COVID-19 in relation to their Child Pugh Score, no significant association was found for child scores A, B, or C. Despite the fact that 55.6% of CLD with COVID-19 vs. 18.2% without COVID-19 were admitted with a child score of A, and 18.5% vs. 60.6% were admitted with a child score of C, 75% of the COVID-19 admissions with CLD that were deceased had a child class of C, compared to 95.5% of CLD admissions without COVID-19.21
Almost 16% CLD patients admitted with COVID-19 had acute on chronic hepatitis. According to the published data, 14%-53% of COVID-19 patients developed hepatic impairment manifested by raised aminotransferases, whereas only 2%-11% were reported to have underlying CLD.22 So, SARS-Cov-2 can independently damage the liver, leading to acute liver failure. COVID-19's mechanism of liver damage is likely complex. SARS-CoV-2 may induce direct hepatotoxicity when it enters cholangiocytes via ACE 2 receptors or indirect hepatotoxicity from systemic dysregulated inflammation, hypoxemia, ischemic damage attributed to coagulopathy, drug-induced hepatic damage or acute insult on pre-existing liver disease.23
Metabolic-associated fatty liver disease (MAFLD) and non-alcoholic steatohepatitis (NASH) had been associated with high morbidity and mortality among patients admitted with COVID-19,23,24 however, associated comorbid conditions like dyslipidemia, obesity, diabetes mellitus and ischemic heart disease had been found as independent risk factors among them. In this regard very less data is available signifying the chronic liver disease caused by hepatitis B and C as independent risk factors for the severity of the disease among COVID-19 patients.7 One of the meta data shows decreased incidence of COVID-19 among cirrhosis patients.8 One large study from USA explored the role of HCV infection on the risk of hospitalisation and all-cause mortality in comparison to COVID-19 hospitalisations without HCV infection, as well as the effect of liver fibrosis stage on these outcomes. Overall hospitalisations were greater among those with HCV, but ICU admission and all-cause death were comparable between those with and without HCV.25 So, a study from a country with the second-highest prevalence of HCV and the highest number of hospitalisations related to chronic liver disease will help us learn more about how chronic liver disease caused by HCV and HBV affects hospitalisations related to COVID-19 and how severe COVID-19 is in people with chronic liver disease caused by viral hepatitis compared to people with other comorbid conditions.
There are a few limitations to the results of this study. First, this observational study may have experienced unmeasured confounding. Second, the progression of COVID-19 to high severity was assessed using a variety of outcomes, including hospital stay, oxygen demand, death, and the need for intensive care. The COVID-19 life-threatening consequences are captured by these metrics, despite the fact that they do not fully reflect disease severity. Thirdly, because only hospitalised patients were included in this analysis, it is impossible to say if the presence of CLD lessens the severity of COVID-19 in outpatients.
CONCLUSION
Chronic liver disease caused by viral hepatitis C and B was significantly less prevalent in patients hospitalised with COVID-19 than in patients hospitalised for non-COVID medical illnesses. Upper gastrointestinal bleeding was the most common cause of death among CLD patients admitted for COVID-19. Patients with COVID-19 and CLD were less likely to die or need admission to intensive care and more likely to be admitted with a moderate illness as compared to those with other comorbidities like diabetes mellitus, cardiovascular disease, and chronic kidney disease.
ETHICAL APPROVAL:
Approval for this study had been taken before the start of this study, from the ethical review board, Department of medical education, Qauid-e-Azam Medical College Bahawalpur, Pakistan; vide number 1072/DME/QAMC Bahawalpur.
PATIENTS' CONSENT:
Informed consent was obtained from patients to publish the data concerning this case.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
JA: Conceptualization.
JA, FQ: Manuscript writing.
JA, MK: Acquisition of data, data analysis.
JA, D-e-S, MK, AYA: Data interpretation.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Tenforde MW, Self WH, Adams K, Gaglani M, Ginde AA, McNeal T, et al. Association between mRNA vaccination and COVID-19 Hospitalization and disease severity. Jama 2021; 326(20):2043-54. doi: 10.1001/jama.2021.19499.
- Aslam J, Rauf ul Hassan M, Fatima Q, Bashir Hashmi H, Alshahrani MY, Alkhathami AG, et al. Association of disease severity and death outcome with vaccination status of admitted COVID-19 patients in delta period of SARS-COV-2 in mixed variety of vaccine background. Saudi J Biol Sci 2022; 103329. doi: 10/1016/j.sjbs.2022.103329.
- World Health Organization. Weekly epidemiological update on COVID-19 [Internet]. [cited 2022 Jun 3]. Available from: https://covid19.who.int
- McConnell MJ, Kondo R, Kawaguchi N, Iwakiri Y. Covid-19 and liver injury: Role of inflammatory endotheliopathy, platelet dysfunction, and thrombosis. Hepatol Commun 2022; 6(2):255-69. doi: 10.1002/hep4.1
- Sumeet K A, Harshad D, Jhon E, Kamath PS. Burden of liver diseases in the world. J Hepatol 2019; 70(1):151-71. doi: 10.1016/j.jhep.2018.09.014.
- Kovalic AJ, Satapathy SK, Thuluvath PJ. Prevalence of chronic liver disease in patients with COVID-19 and their clinical outcomes: A systematic review and meta-analysis. Hepatol Int 2020; 14(5):612-20. doi.org/10.1007/s12072-020- 10078-2.
- Martinez MA, Franco S. Impact of COVID-19 in Liver disease progression. Hepatol Commun 2021; 5(7):1138-50. doi: 10.1002/hep4.1745.
- Philips CA, Kakkar K, Joseph M, Yerol PK, Ahamed R, Rajesh S, et al. Critically ill COVID-19 patient with chronic liver disease-insights into a comprehensive liver intensive care. J Clin Transl Hepatol 2021; 9(4):576-86. doi: 10.14218/JCTH. 2020.00110.
- Wedemeyer H, Dore GJ, Ward JW. Estimates on HCV disease burden worldwide - Filling the gaps. J Viral Hepat 2015; 22(s1):1-5. doi: 10.1111/jvh.12371.
- Alberts CJ, Clifford GM, Georges D, Negro F, Lesi OA, Hutin YJF, et al. Worldwide prevalence of hepatitis B virus and hepatitis C virus among patients with cirrhosis at country, region, and global levels: A systematic review. Lancet Gastroenterol Hepatol 2022; 7(8):724-35. dx.doi.org/10. 1016/S2468-1253(22)00050-4.
- World Health Organisation. Global hepatitis report, 2017. [Internet]. [cited 2022 June]. Available from https://www. who.int/publications/i/item/2989241565455.
- Abbas Z, Abbas M. The cost of eliminating hepatitis C in Pakistan. Lancet Glob Heal 2020; 8(3):e323-4. doi.org/10. 1016/S2214-109X(20)30036-X.
- Khan TS, Farhat R. Hepatitis B seropositivity among chronic liver disease patients in Hazara division Pakistan. J Ayub Med Coll Abbottabad 2003; 15(3):54-5.
- Khan TS, Rizvi F, Rashid A. Hepatitis C seropositivity among chronic liver disease patients in Hazara, Pakistan. J Ayub Med Coll Abbottabad 2003; 15(2)53-5.
- Sundaramurthy R, Balasubramanian S, Ganesan V, Aggarwal P, Parvataneni T, Jyothi Ramachandran Nair DP, et al. Clinical and laboratory factors in predicting mortality among COVID-19 RT-PCR positive patients: A retrospective observational study from a tertiary care centre. Cureus 2021; 13(11). doi: 10.7759/cureus. 19791.
- Noor MT, Manoria P. Immune dysfunction in cirrhosis. J Clin Transl Hepatol 2017; 5(1):50-8. doi: 10.14218/JCTH.2016. 00056.
- Tsoris A, Marlar CA. Use of the child pugh score in liver disease. 1-4 p. www.statpearls.com/ArticleLibrary/view article/19534.
- Baz AAM, Mohamed RM, El-kaffas KH. Doppler ultrasound in liver cirrhosis: Correlation of hepatic artery and portal vein measurements with model for end-stage liver disease score in Egypt. Egypt J Radiol Nucl Med 2017; 36(4):725-30. doi: 10.7863/ultra.16.03107.
- Philips CA, Rela M, Soin AS, Gupta S, Surendran S, Augustine P. Critical update on the diagnosis and management of COVID-19 in advanced cirrhosis and liver transplant recipients. J Clin Transl Hepatol 2021; 9(6): 947-59. doi: 10. 14218/JCTH.2021.00228.
- Mani I, Alexopoulou A. Recent challenges facing patients with preexisting chronic liver disease in the era of the COVID-19 pandemic. Ann Gastroenterol 2021; 34(5): 625-33. doi: 10.20524/aog.2021.0628.
- Cerbu B, Grigoras ML, Bratosin F, Bogdan I, Citu C, Bota AV, et al. Laboratory profile of COVID-19 patients with hepatitis C-related liver cirrhosis. J Clin Med 2022; 11(3). doi: 10. 3390/jcm11030652.
- Nasa P, Alexander G. COVID-19 and the liver: What do we know so far? World J Hepatol 2021; 13(5):522-32. doi: 10.4254/wjh.v13.i5.522.
- Crisan D, Avram L, Grapa C, Dragan A, Radulescu D, Crisan S, et al. Liver injury and elevated fib-4 define a high-risk group in patients with COVID-19. J Clin Med 2022; 11(1). doi: 10.3390/jcm11010153.
- Sarin SK, Choudhury A, Lau GK, Zheng MH, Ji D, Abd-Elsalam S, et al. Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS study (APASL COVID-19 liver injury spectrum study). Hepatol Int 2020; 14(5):690-700. doi.org/10. 1007/s12072-020- 10072-8.
- Butt AA, Yan P, Chotani RA, Shaikh OS. Mortality is not increased in SARS-CoV-2 infected persons with hepatitis C virus infection. Liver Int 2021; 41(8):1824-31. doi: 10. 1111/liv.14804.