An Innovation in the Technique of Recipient Hepatectomy in Living Donor Liver Transplantation
By Shams Uddin, Kaleem Ullah, Abdul Wahab Dogar, Syed Hasnain Abbas, Siraj Khoso, Bilal AhmedAffiliations
doi: 10.29271/jcpsp.2022.08.1060ABSTRACT
Recipient hepatectomy is a challenging surgical procedure. Coagulopathy, multiple collaterals, and dense adhesions secondary to previous spontaneous bacterial peritonitis in cirrhotics are the major contributing factors. However, the appropriate recipient hepatectomy technique can limit the massive blood loss and minimize the operative time. The hepatoduodenal dissection has a key role in recipient hepatectomy. The hilar structures of partial graft in live donor liver transplantation (LDLT) usually have a short length and a small caliber. The concerning task in LDLT recipient hepatectomy is to preserve the integrity, quality, and adequacy of hilar structures for successful implantation. The high hilar dissection technique is usually practiced for getting the adequate length of hilar structures. However, the problems with high hilar dissection inducted the authors to tailor the technique over time. In this report, a modified technique of recipient hepatectomy characterised by the artery-first approach is described. This technique is good in terms of preventing arterial dissection and minimising the anhepatic phase.
Key Words: Recipient hepatectomy, Hepatoduodenal dissection, Liver transplantation, Technique.
INTRODUCTION
In liver transplantation, the recipient's hepatectomy is a challenging task. It always requires meticulous techniques to minimize blood loss and prevent arterial dissection. During hepatoduodenal dissection various hilar structures, i.e. hepatic artery, portal vein, and bile duct are skeletonised.1 Every transplant team prefers their technique for recipient hepatectomy.2
The hilar structures of partial graft in live donor liver transplantation (LDLT) usually have a short length and small caliber. The concerning task in LDLT recipient hepatectomy is to preserve the integrity, quality, and adequacy of hilar structures for successful implantation.3 The high hilar division provides adequate length and vascularised bile ducts. It allows tension-free biliary anastomosis. Moreover, it also facilitates multiple anastomoses in a required situation. Excessive biliary ducts skeletonisation should be avoided as it may interrupt the biliary ducts' blood supply which can result in an increased incidence of post-transplant biliary complications.2
The hepatic artery branches and any accessory artery should also be preserved.4 Here, in this report, the authors describe their tailored recipient hepatectomy technique along with hepatoduodenal dissection characterised by the artery-first approach and a portal flow preserving high hilar biliary duct division technique.
The Technique of Recipient Hepatectomy:
The Mercedes Benz incision is the most practiced incision worldwide for recipient hepatectomy.5 This incision is preferred in the recipients with minimal ascites (Figure 1a). Whereas, an Inverted-T incision can be used in the recipients with moderate to massive ascites (Figure 1b). In slim recipients, a reverse -L incision is good enough for appropriate exposure.
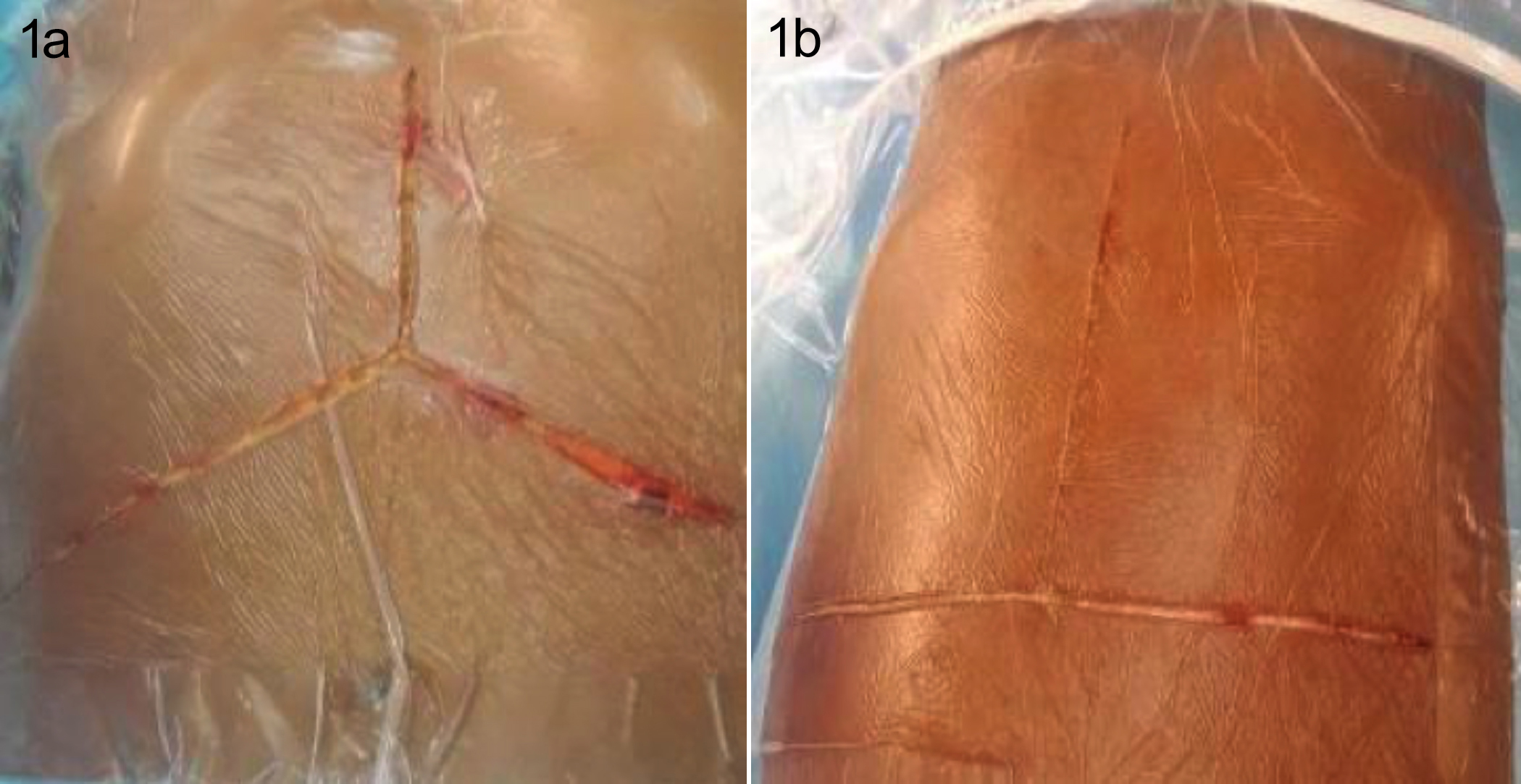 Figure 1a: Mercedes incision on the right side. 1b: Shows an inverted T incision on the left side.
Figure 1a: Mercedes incision on the right side. 1b: Shows an inverted T incision on the left side.
After entering the peritoneal cavity, the falciform ligament is divided and the umbilical vein is secured with a tie. After detailed visceral inspection, mobilisation of the left lobe of the liver is done by dividing the left coronary and triangular ligaments with monopolar diathermy. Then hepato-gastric ligament is divided while taking care of accessory or replaced left hepatic artery (LHA) arising from the left gastric artery (LGA). It is secured with a vascular bulldog clamp and transected near the liver surface to get the appropriate length.
In patients with severe portal hypertension and multiple collaterals, hepatoduodenal ligament dissection is preferred first before right lobe mobilisation. While in routine, the right lobe is mobilised first before hepatoduodenal dissection. The right triangular and coronary ligament is divided and adrenal gland is dissected from the liver and the Inferior vena cava (IVC) is exposed. Then small caudate branches are secured with ties or clips and mobilisation is continued. After dividing the Makuuchi ligament, space is created between the right hepatic vein (RHV) and middle hepatic vein (MHV), and RHV is slinged. Care is taken not to tear the liver capsule during mobilisation as it bleed profusely. The portocaval shunting is done rarely in very difficult hepatectomy.
The goal of hepatoduodenal ligament dissection is to get good length vascular structures and to avoid arterial intimal injury. An adequate hepatoduodenal dissection also ensures the provision of the maximum number of biliary ducts with adequate blood supply.
Early division of cystic duct and cystic artery with total or partial cholecystectomy facilitates hepatoduodenal dissection (Figure 2a). The hepatoduodenal dissection is started laterally and carried medially over the upper border of the duodenum by incising the peritoneum and dividing the small vessels (Figure 2b).
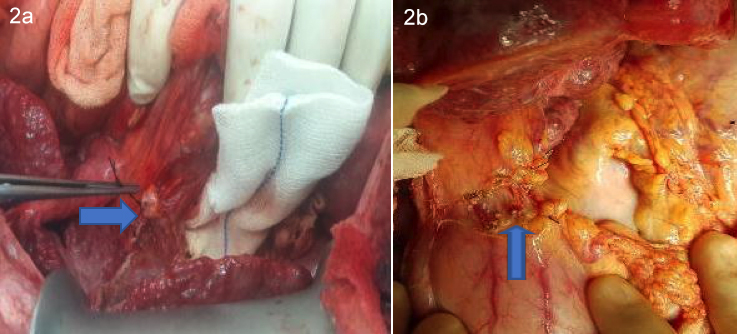 Figure 2a: Arrow marks a tied cystic duct stump. 2b: Arrow marks the divided peritoneal covering over the hepatic artery.
Figure 2a: Arrow marks a tied cystic duct stump. 2b: Arrow marks the divided peritoneal covering over the hepatic artery.
Palpation of the common hepatic artery (CHA) with the thumb and index finger guides the position of the artery. Peritoneal covering over the pulsated area is removed after the division of the right gastric artery (RGA). The RGA artery is superficial in position, so it should not be confused with the hepatic artery. Then hepatoduodenal lymph node, which acts as a landmark is identified. Partial mobilisation or removal of this lymph node facilitate identification of the CHA. After the skeletonisation of the CHA, space is created around the artery and encircled with a vascular sling. A vascular bulldog clamp is applied to the artery to avoid intimal dissection by interrupting the forward blood flow. At this point, the gastroduodenal artery (GDA) is looked in the triangular area formed laterally by the common bile duct (CBD), medially by CHA, and the inferiorly by the duodenum. GDA is also encircled with a vascular sling. Transection of GDA is not always necessary, however, its division gives length to the hepatic artery and also avoided the steal syndrome. Before division of GDA, vascular bulldog clamp is applied on it and distal pulsation is confirmed.
The dissection is then continued upward and the left hepatic artery (LHA) and segment IV branch are divided near the hilum (Figure 3a and 3b). The right hepatic artery (RHA) is left undisturbed along with the CBD. After the division of the LHA, the left portal vein (LPV) is exposed by dissecting the fibrous tissues. It is termed as undressing the LPV (Figure 4).
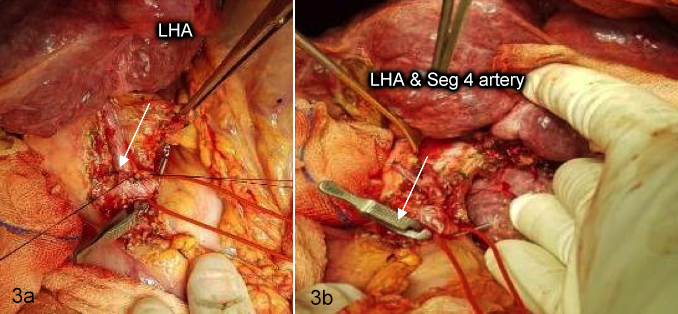 Figure 3a: Arrow marking LHA while bulldog clamp is applied on. 3b: Arrow marking the divided LHA and Seg 4 artery
Figure 3a: Arrow marking LHA while bulldog clamp is applied on. 3b: Arrow marking the divided LHA and Seg 4 artery
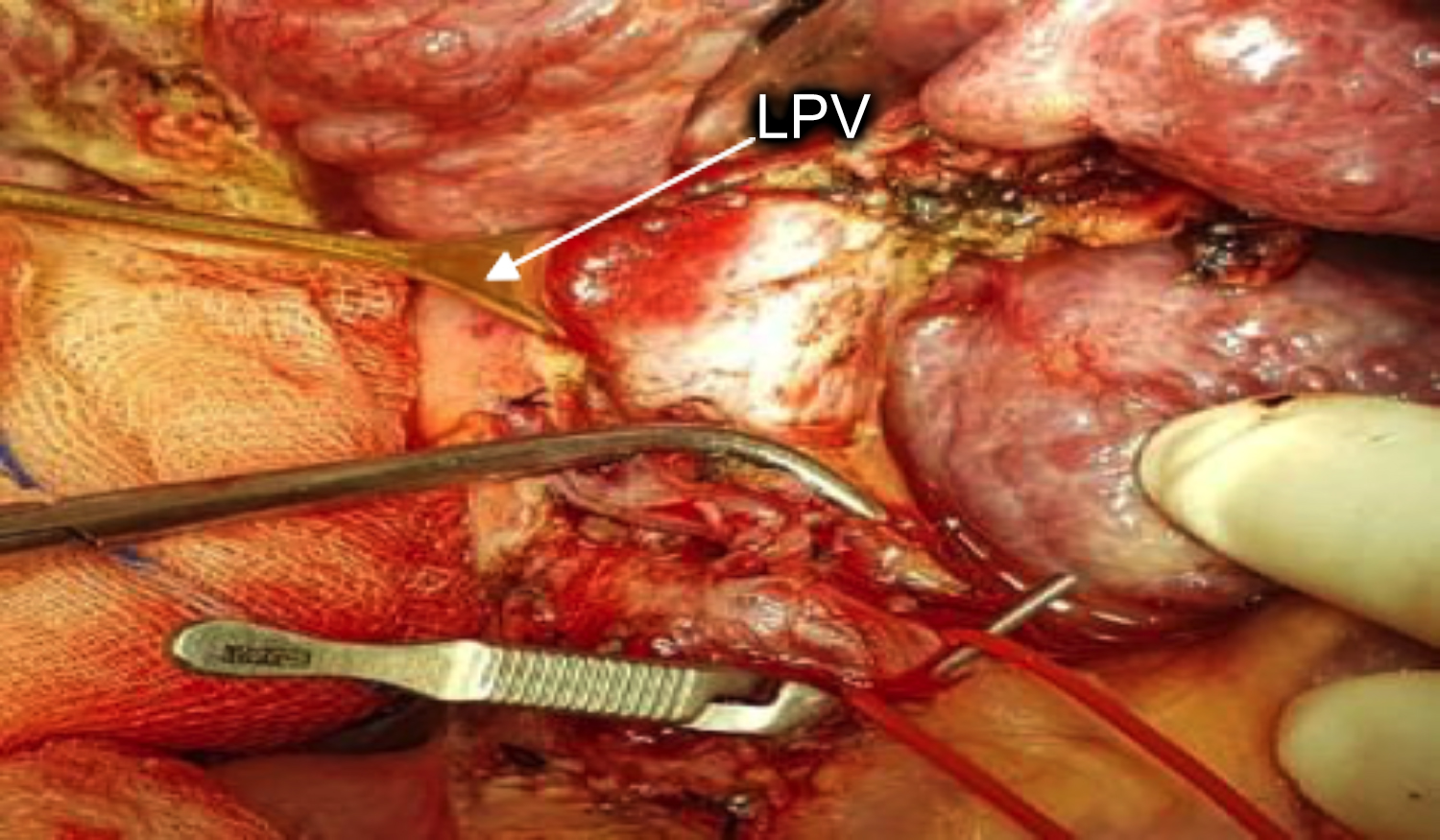 Figure 4: Arrow pointing towards left portal vein (LPV).
Figure 4: Arrow pointing towards left portal vein (LPV).
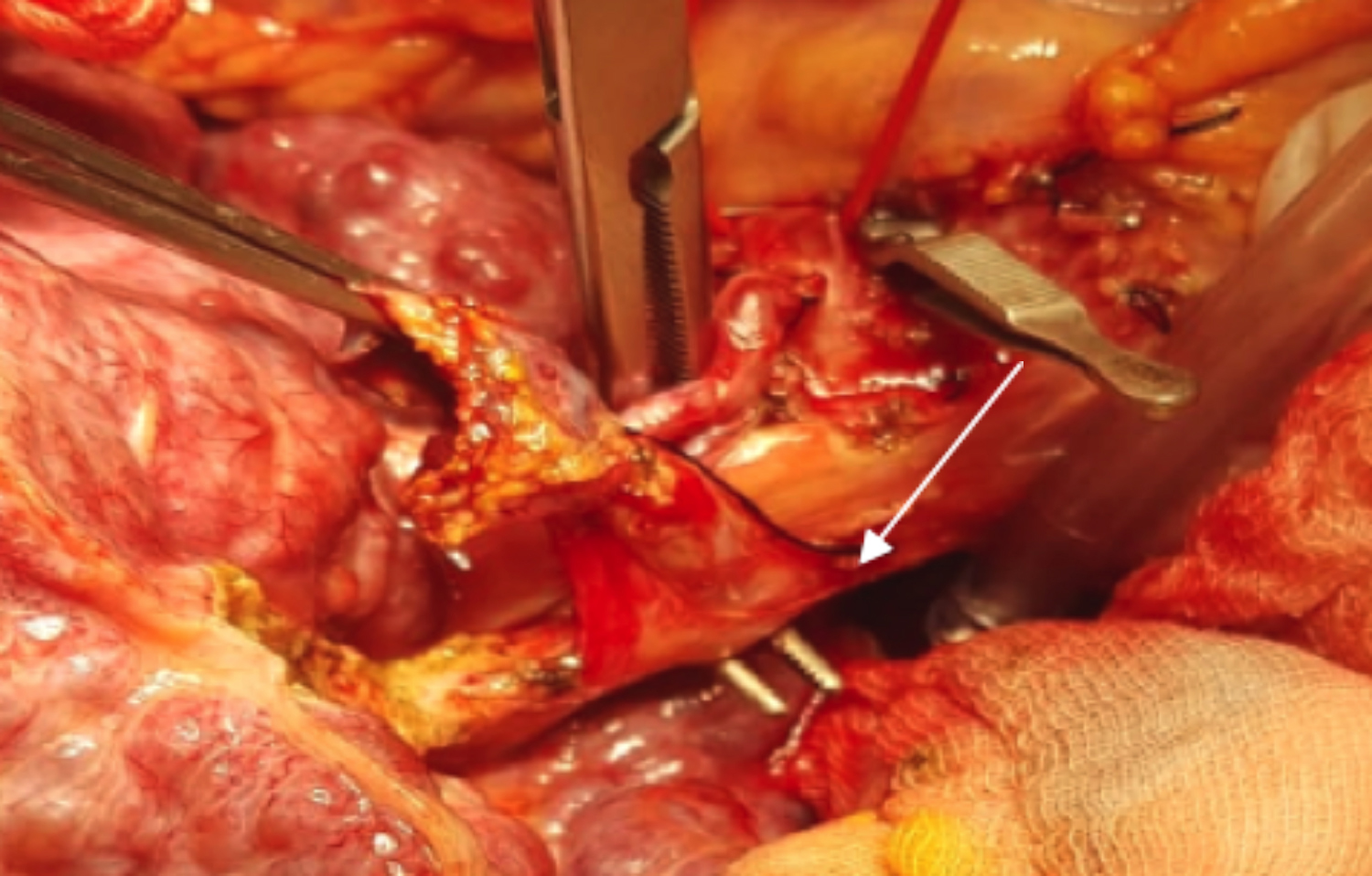 Figure 5: Arrow showing right-angled forcep through the tunnel.
Figure 5: Arrow showing right-angled forcep through the tunnel.
After exposing the LPV, the dissection is continued over the main portal vein (MPV). After undressing the MPV medially, the tissues over the MPV are held with an eye lid retractor and the atraumatic right-angled forcep is passed from medially to laterally creating a tunnel between the CBD and MPV, while taking care of replaced RHA. The CBD along with the right hepatic artery is then slinged. This is called the tunnel technique (Figure 5).
Then the remaining tissue over the MPV is dissected from the right side and the MPV is also slinged.
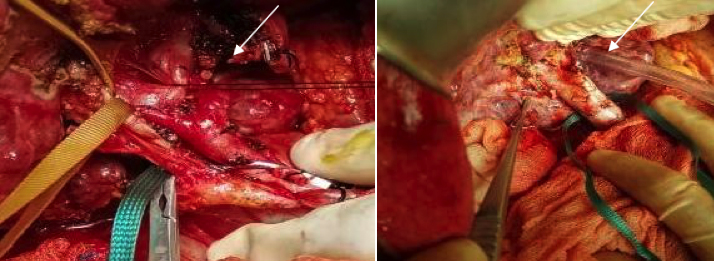 Figure 6a: Arrow is showing ligation of LPV with a silk suture. 6b: Arrow is showing ligated LPV and transected multiple biliary ducts.
Figure 6a: Arrow is showing ligation of LPV with a silk suture. 6b: Arrow is showing ligated LPV and transected multiple biliary ducts.
The vascular clamp is then applied over the MPV, and LPV is divided (Figure 6a). The RHA is then ligated near the hilum and the biliary tree transected as high as possible to get multiple ducts (Figure 6b). An early division of LPV facilitates the high division of the biliary tree. MPV clamping during biliary ducts transaction preventes vision-obscuring secondary to bleeding which can misguide the transaction. After the division of the biliary tree, hemostasis is secured over the cut liver surface of the liver with a 4-0 proline and a bull-dog clamp is applied over the cut surface of the biliary tree. The portal vein clamp is removed and portal flow is restored with the right portal vein (RPV) which reduces the anhepatic phase.
The RPV is divided when the recipient team gets a call from the donor’s team. The RHV is divided after RPV division and the remaining liver i.e. left and caudate lobes are mobilised. MHV and left hepatic vein are divided and explant hepatectomy is completed.
Suture ligation is preferred for hepatoduodenal dissection along with adjusted low voltage (25 Watt) monopolar and bipolar diathermy for dissecting and hemostasis purposes. Cell savers are not used routinely.
DISCUSSION
Preserving the maximum number of hilar structures with appropriate length is of utmost importance for successful implantation.5 Initially, the authors used to start the hepatoduodenal dissection near the hilum. The philosophy was to get appropriate length hilar structures and avoid damage to the proximal structures in the hepatoduodenal ligament. Conversely, a few problems were faced with this technique. Working near the hilum was difficult due to the narrow space containing collaterals that often bleed profusely. Hemorrhage from the capsular tears was also difficult to handle. Intimal dissection was observed frequently with high hilar dissection in the absence of proximal arterial control. This was also observed by Kayaalp et al. and they were the ones who first introduced this artery-first approach.5 So, the authors switched the hepatoduodenal dissection technique to the artery-first approach targeting the CHA artery for the proximal control. This technique also facilitates multiple, well-vascularised, and tension-free biliary anastomoses. An early division of LPV is important to ensure high division of biliary ducts in a required situation.. This technique has also got the advantage of decreasing the duration of the anhepatic phase as continuous portal supply is maintained through the RPV.
Moreover, the artery is one of the key structures in the hepatoduodenal ligament having maximum variations.6 Major branches of the hepatic artery like GDA along with accessories arteries should be preserved as any of these can be potentially used during implantation.
CONCLUSION
The technique of recipient hepatectomy can be tailored according to the situation. The authors recommend the described technique for better results as it minimised the frequency of arterial intimal injury, reduced the anhepatic phase, and preserved the maximum number of hilar structures.
ETHICAL APPROVAL:
Ethical approval was obtained before conducting the research work from the ethical committee of the Liver transplant department Pir Abdul Qadir Shah Jeelani Institute of Medical Sciences, Gambat, Pakistan.
PATIENTS’ CONSENT:
Not applicable in this study.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
SU: Study design
KU, BA: Manuscript writing
AWD: Study supervision
SHA: Literature search
SK: Manuscript revision
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Vellar ID. Preliminary study of the anatomy of the venous drainage of the intrahepatic and extrahepatic bile ducts and its relevance to the practice of hepatobiliary surgery. ANZ J Surg 2001; 71(7):418-22. doi: 10.1046/j.1440- 1622.2001.02150.x.
- Soejima Y, Fukuhara T, Morita K, Yoshizumi T, Ikegami T, Yamashita Y, et al. A simple hilar dissection technique preserving maximum blood supply to the bile duct in living donor liver transplantation. Transplantation 2008; 86(10):1468-9. doi: 10.1097/TP.0b013e318188d4dc.
- Dulundu E, Sugawara Y, Kishi Y, Akamatsu N, Kokudo N, Makuuchi M, et al. Phrenic vein dissection in partial liver graft harvesting. Hepatogastroenterol 2006; 53(71): 778-80.
- Mori K, Nagata I, Yamagata S, Sasaki H, Nishizawa F, Takada Y, et al. The introduction of microvascular surgery to hepatic artery reconstruction in living-donor liver transplantation — its surgical advantages compared with conventional procedures. Transplantation 1992; 54(2): 263-8. doi: 10.1097/00007890-199208000-00014.
- Kayaalp C, Tolan K, Yilmaz S. Hepatoduodenal ligament dissection technique during recipient hepatectomy for liver transplantation: How I do it? World J Transplant 2016; 6(2):272-7. doi: 10.5500/wjt.v6.i2.272. PMID: 27358772.
- Ullah K, Dogar AW, Uddin S, Hasnain S, Ahmad B, Ghaffar A. Frequency and outcome of hepatic arterial thrombosis in recipients of living donor liver transplantation. J Coll Physicians Surg Pak 2021; 31(8):897-902. doi: 10.29271/ jcpsp.2021.08.897.