Accuracy of Diffusion Weighted Imaging in Assessment of Pelvic Lymphnode Metastasis in Patients with Endometrial Cancer
By Imrana Masroor, Shaista Afzal, Hina PathanAffiliations
doi: 10.29271/jcpsp.2023.07.738ABSTRACT
Objective: To evaluate the sensitivity and specificity of diffusion-weighted imaging in determining metastatic pelvic lymph nodes in patients of endometrial cancer and comparing its accuracy with contrast-enhanced sequence of MRI, taking histopathology as gold standard.
Study Design: Retrospective Study.
Place and Duration of the Study: Department of Radiology, the Aga Khan University Hospital Karachi, from January to December 2021.
Methodology: Fifty-eight adult females with biopsy proven endometrial carcinoma and complete medical records were included through convenience sampling. Patients who did not have complete medical records were excluded. Studied variables included signal characteristics of lymph nodes and their short axis diameter. The sensitivity and specificity of DWI and contrast-enhanced MRI for evaluation of diseased lymph nodes were calculated using histopathology as the gold standard.
Results: Among 58 patients with histopathologically proven endometrial cancer, 14 had metastatic lymphadenopathy. DWI-weighted imaging in the evaluation of metastatic and non-metastatic lymph nodes had sensitivity of 81.1% while specificity, the positive and negative predictive value of 88.8%, 72.2%, and 82.5% and on contrast-enhanced imaging as 66.6%, 58.1%, 35.7%, and 83.3%, respectively.
Conclusion: The DWI shows better accuracy in evaluation and discrimination between metastatic and non-metastatic lymph nodes as compared to contrast-enhanced MRI examination for the evaluation of diseased lymph nodes in patients with endometrial cancer.
Key Words: DWI, Contrast-enhanced MRI, Lymph node, Endometrial cancer.
INTRODUCTION
Endometrial cancer has become the sixth most common cancer in females and the leading gynaecological cancer in first-world countries.1,2 Endometrial cancer is usually diagnosed at the earlier stage. It is more common in females who are above 50 years. Endometrial biopsy is done to grade and evaluate histological subtypes. The preoperative tumour staging is performed according to the International Federation of Gynaecology and Obstetrics (FIGO) guidelines and it depends upon intraoperative staging that includes total abdominal hysterectomy and removal of both ovaries along with fallopian tubes. The peritoneal lavage, para-aortic and pelvic lymph node dissection are also done.3
Different prognostic factors have impact on survival of patient including how far is the involvement of myometrium, diseased lymph nodes, and grade of tumour.4,5
Magnetic resonance imaging (MRI) has emerged as an effective modality for the preoperative evaluation of endometrial cancer in terms of computation of myometrial invasion, involvement of cervical stroma, and pelvic lymph node metastasis.6,7
DW imaging can be used as an alternative to contrast-enhanced imaging reducing scan time and cost of contrast. It was hypothesised that DW imaging has better sensitivity and specificity in evaluating metastatic lymph nodes than contrast-enhanced imaging.
There is no local data available on the comparison of both MRI sequences for loco-regional lymphadenopathy. This has important implications for local protocol development.
The aim to conduct this study was to evaluate the effectiveness of DW imaging in comparison to contrast-enhanced MRI (CE-MRI) for the evaluation of metastatic pelvic lymph nodes in patients with endometrial cancer.
METHODOLOGY
This retrospective study was conducted in the Radiology Department of the Aga Khan University Hospital, from January to December 2021. The approval for exemption was taken from the Hospital Ethical Review Committee (Reference 2022-7710- 22091). The data were retrospectively collected from the workstation having a facility of picture archiving and communication system (PACS), so the need for informed consent was abjured.
Total 58 patients having complete medical records with biopsy-proven endometrial neoplasia were elected through convenience sampling.
All those patients had surgery with complete removal of the uterus and adnexal structures and loco-regional lymph nodes followed by histopathological staging. All other patients having history of treated endometrial cancer or other malignancy, recent history of pelvic inflammatory disease and hysterectomy were excluded from the study. The MR scan of pelvis was performed on 1.5 Tesla Siemens machine. T2 axial, T2 coronal, and T2 sagittal and fat-suppressed T1 post-contrast images in axial, coronal and sagittal planes were obtained. T1 fat-sat axial images were also acquired axial DW imaging with corresponding ADC mapping done at b-value of 50, 400, and 800 sec/mm2. Bright signals on DWI and dark signals on ADC were considered as diffusion-positive nodes. The lymph node on post- contrast images showing enhancement equal or more than the myometrium was considered enhanced lymph node. The diffusion-positive pelvic lymph nodes and histopathologically proven diseased lymph nodes were calculated as true positives. Those lymph nodes which were DWI positive but histopathology revealed benign findings were false-positive lymph nodes. The reciprocal was true for false-negative metastatic lymph nodes. The same method was used for contrast-enhanced lymph nodes. The hospital database was retrospectively reviewed by two radiologists on the workstation. Radiologists were aware of endometrial cancer diagnosis but were blinded to histopathological diagnosis of pelvic lymph nodes, subtype of endometrial cancer and depth of myometrial incursion. In case of difference in opinion, a neutral opinion was obtained from 3rd radiologist. Diffusion positivity and negativity depend upon the signal characteristics of lymph nodes.
Pelvic lymph nodes evaluated were the common iliac, along its terminal branches the internal and external iliac chain, the obturator and pre-sacral nodes. The sensitivity, specificity, and positive and negative predictive values were calculated for both sets to determine and compare the accuracies.
Patient-based analysis was performed with reference to consensus verdict. Sensitivity, specificity, positive predictive value and negative predictive value, and accuracy were calculated by means of standard statistical formulas. Chi-square test was used to determine the statistical significance of differences between DWI and CE-MRI interpretations. A p-value of <0.05 was regarded as statistically significant.
RESULTS
The mean age of patients was 55 ± 15 years. All patients had presented with postmenopausal bleeding which later had biopsy. Most of the patients were obese having multiple pregnancies (n=35, % 60.34%) and past history of taking birth control pills (n=28, 48.27%).
Among total 58 patients who had endometrial carcinoma, 18 were diffusion-positive and 37 patients were diffusion-negative in regard to lymphadenopathy. On contrast-imaging, 28 patients showed enhanced lymph nodes and 5 patients were negative on CE-MRI. Correlating with the histopathology of lymph nodes, the number of true positive cases was more on DWI as compared to CE-MRI with a ratio of 13:5, respectively. The true negative cases on DWI were 37 and on CE-MRI were 25. Thus the sensitivity and specificity in determining the pathological lymph nodes on DWI sequence was 81.1% and 88.8% as compared to CE-MRI which was 66.6% and 58.1%, proving it to be a more sensitive sequence of MRI for evaluation of metastatic lymphadenopathy with a positive predictive value of 72.2% and negative predictive value of 92.5% (Table I).
Out of the total, 14 patients had lymphadenopathy on histopathology, the distribution of involved and uninvolved lymph nodes correlating with histopathology results are given in Table I on DWI and CE-MRI. The incidence of the obturator and external iliac lymph nodes was more common at 13% and 10%, respectively. The internal iliac lymph nodes 8% and pre-sacral lymph nodes were 2%.
Twelve patients having metastatic lymphadenopathy showed more than half of myometrial invasion, on diffusion-weighted images suggesting a linear relationship regarding the spread of locally advanced disease.
Table I: Comparative accuracy of diffusion-weighted imaging (DWI) and contract-enhanced (CE) MRI in metering metastatic pelvic lymph nodes in endometrial cancer.
|
|
Number of patients and % |
|
|
Performance of DWI |
Performance of CE-MRI |
|
|
True positive (n) |
13 |
10 |
|
True negative (n) |
37 |
25 |
|
False positive (n) |
05 |
18 |
|
False negative (n) |
03 |
05 |
|
Sensitivity % |
81.1 |
66.6 |
|
Specificity % |
88.8 |
58.1 |
|
Positive predictive value % |
72.2 |
35.7 |
|
Negative predictive value % |
92.5 |
83.3 |
DISCUSSION
Developed countries are facing endometrial cancer as a common gynaecological malignancy and fourth on rank in the United states.8,9 MRI examination is modality of choice for staging and evaluation of the disease particularly for uterine malignancy.
This study aimed to establish the role of MRI, using DW, ADC, and post-contrast images in evaluating pelvic lymph node involvement with regard to treatment plans and assessing the prognosis of the disease. The calculations of specificity and sensitivity of diffusion-weighted imaging in predicting lymph node involvement was the main aim of this study.
Choi et al. outlined the ranges for precision of MRI in uterine cancer for the evaluation of diseased lymph nodes. The overall MRI accuracy without sequence discrimination had sensitivity ranging from 30.3%–70.6% and specificity as 74.0%–92.6%, respectively.10 In another study by Jung et al. the diseased lymph nodes had sensitivity of 24.3% detected on MRI and specificity of 96.3%.11
In the present study, the accuracy of DW MRI for the evaluation of diseased lymph nodes was 81% (sensitivity) and 88% (specificity) which is comparable with the study done by Arian and colleagues specifically done to evaluate the role of DWI in pelvic lymphadenopathy.12 They took 33 pathologically proven patients of endometrial cancer and found all metastatic lymph nodes showing diffusion restriction and 19.3% false positive lymph nodes. This study in comparison have a larger sample size and less false positive lymph nodes (8.6%).
The involvement of pelvic lymph nodes according to the anatomical region is also different in the case of endometrial carcinoma. According to literature, some surgical studies suggest that among the involved pelvic nodes, the most common location is found to be the obturator and external iliac chain.13,14 The findings of this study also correlate with the involvement of the diseased lymph nodes more commonly at this location.
In addition, the authors also assessed the myometrium involvement, and its depth of invasion in this study on diffusion-weighted images which showed the majority of the patients having metastatic lymphadenopathy have more than half of myometrial invasion as shown in Figures 1 and 2.
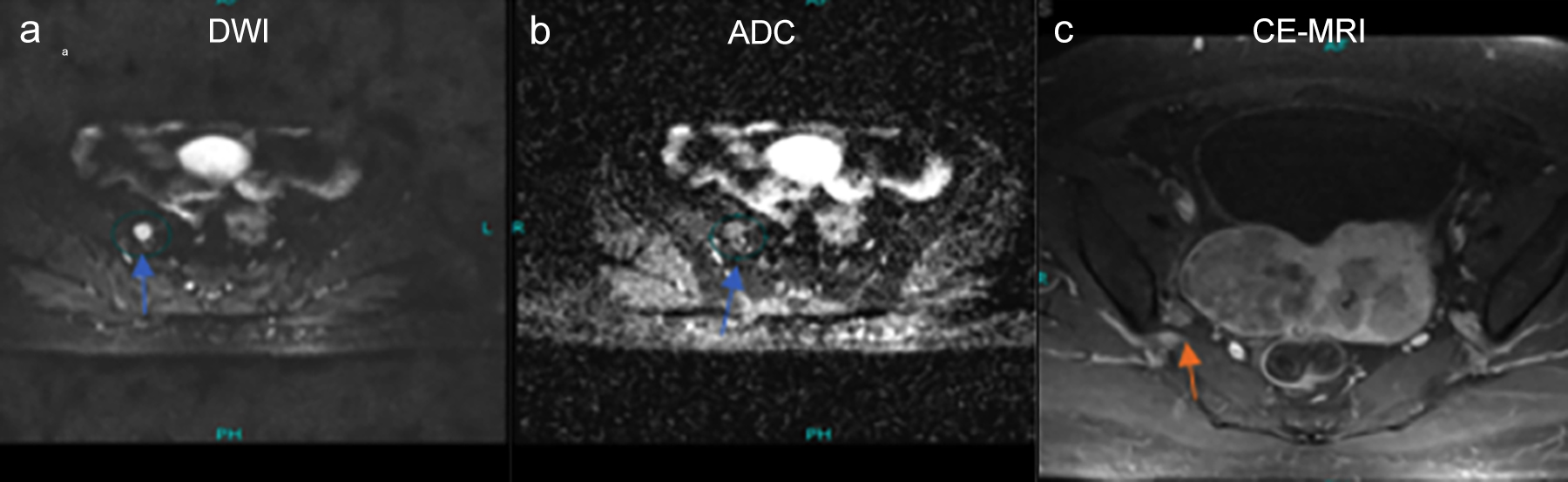 Figure 1: A 58-years old female with endometroid carcinoma, the axial DWI and ADC images are showing the right pelvic lymph node with round morphology and restriction on diffusion-weighted images marked with a green circle and blue arrow (image a,b). While contrast enhancement of the corresponding lymph node is less as compared to the enhancing myometrium on post-contrast axial image black arrow (c).
Figure 1: A 58-years old female with endometroid carcinoma, the axial DWI and ADC images are showing the right pelvic lymph node with round morphology and restriction on diffusion-weighted images marked with a green circle and blue arrow (image a,b). While contrast enhancement of the corresponding lymph node is less as compared to the enhancing myometrium on post-contrast axial image black arrow (c).
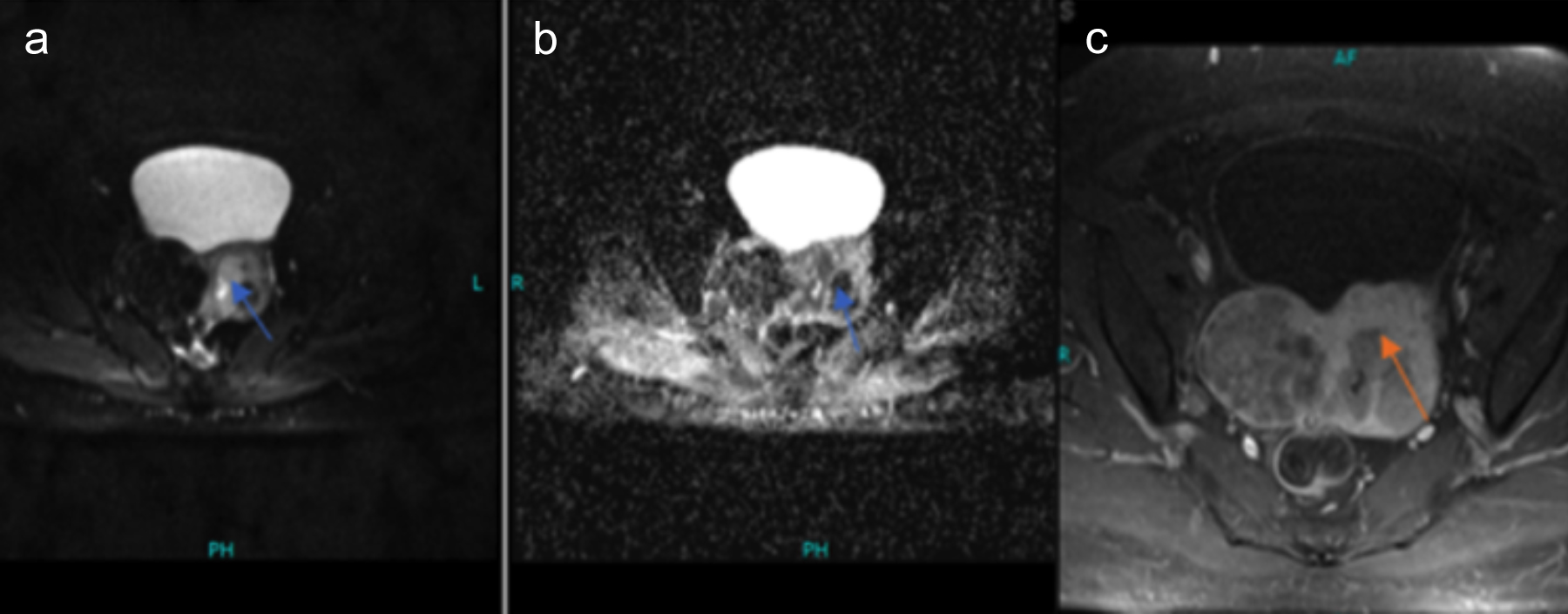 Figure 2: Similar patient with endometroid carcinoma showing more than half of myometrial invasion on axial DWI and corresponding ADC images (image a,b) marked by blue arrow. While post-contrast axial image (c) is also showing com parable involvement of myometrium marked by black arrow.
Figure 2: Similar patient with endometroid carcinoma showing more than half of myometrial invasion on axial DWI and corresponding ADC images (image a,b) marked by blue arrow. While post-contrast axial image (c) is also showing com parable involvement of myometrium marked by black arrow.
Retrospectively, it was also concluded that the majority of the patients having locally advanced endometrioid carcinoma as a common histologic subtype accounting for 85-90% of the cases. It shows slow progression and have good prognosis.15 The rest of the histopathology includes clear, serous carcinoma and others.16
This study has a few limitations. It was done retrospectively, with a small sample size. Secondly, this study was carried out in a single institution so results cannot be generalised. In future multicentre studies with larger sample sizes should be conducted so the results can be generalised.
Despite this, the primary strength of the study is precise assessment of DWI in evaluating the loco-regional lymphadenopathy in endometrial cancer patients in comparison to CE-MRI which can obviate the need of contrast study which itself has many prerequisites and contrast-related side effects. In addition to intrauterine involvement of disease, the DWI also showed comparable effectiveness for evaluating the depth of myometrial invasion. So, in future more studies can be done on a larger scale and consensus should be made to include MR-DWI as an effective sequence in comparison to contrast study, which will not only be cost-effective but also save time and contrast hazards.
CONCLUSION
Diffusion-weighted images are more sensitive and specific in comparison to contrast-enhanced MRI in endometrial cancer for the evaluation of local metastatic lymphadenopathy. There is an additional benefit of DW imaging in evaluating the depth of myometrium invasion thus obviating the need for contrast imaging in patients with endometrial cancer.
ETHICAL APPROVAL:
The present study was approved by the institutional ethics review committee of the Aga Khan University Hospital with Registration Number 2022-7710-22091.
PATIENTS’ CONSENT:
The need of informed consent was negated due to the retrospective nature of the study.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
IM, SA, HP: Contributed equally to collecting data, manuscript formatting, and reviewing the manuscript.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, et al. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: Diagnosis, treatment and follow-up. Int J Gynecologic Cancer 2016; 26(1). dx.doi.org/10.1097/IGC.0000000000000609.
- Andreano A, Rechichi G, Rebora P, Sironi S, Grazia Valsecchi M, Galimberti S. MR diffusion imaging for preoperative staging of myometrial invasion in patients with endometrial cancer: A ystematic review and meta-analysis. Eur Radiol 2014; 24(6):1327-38. doi.org/10. 1007/s00330-014-3139-4.
- Amant F, Mirza MR, Creutzberg CL. Cancer of corpus uteri. Int J Gynecol Obstet 2012; 119 (Suppl 2):S110-S7. doi.org/10.1590/0100-3984.2018.0054.
- Rechichi G, Galimberti S, Signorelli M, Perego P, Grazia Valsecchi M, Sironi S. Myometrial invasion in endometrial cancer: Diagnostic performance of diffusion-weighted MR imaging at 1.5-T. Eur Radiol 2010; 20(3):754-62. doi.org/10.1007/s00330-009-1597-x.
- Sala E, Rockall A, Kubik-Huch RA. Advances in magnetic resonance imaging of endometrial cancer. Eur Radiol 2011; 21:468-73. doi.org/10.1007/s00330-009-1597-x.
- Beddy P, Moyle P, Kataoka M, Yamamoto AK, Joubert I, Lomas D, et al. Evaluation of depth of myometrial invasion and overall staging in endometrial cancer: Comparison of diffusion-weighted and dynamic contrast-enhanced MR imaging. Radiol 2012; 262(2):530-7. doi.org/10.1148/ radiol. 11110984.
- Rauch GM, Kaur H, Choi H, Ernst RD, Klopp AH, Boonsirikamchai P, et al. Optimization of MR imaging forpretreatment evaluation of patients with endometrial and cervical cancer. Radiographics 2014; 34(4):1082–98. doi.org/10.1148/rg.344140001.
- Brand A, Hammond I, Cheuk R. Clinical practice guidelines for the treatment and management of endometrial cancer. Cancer Council 2020. doi.org/10.1186/s13244-022- 01218-3.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70(1):7-30. doi.org/10.3322/ caac. 21590.
- Choi HJ, Roh JW, Seo SS, Lee S, Kim JY, Kim SK, et al. Comparison of the accuracy of magnetic resonance imaging and positron emission tomography/computed tomography in the presurgical detection of lymph node metastases in patients with uterine cervical carcinoma: A prospective study. Cancer 2006; 106(4):914-22. doi. org/10.1002/cncr.2164.
- Jung W, Park KR, Lee KJ, Kim K, Lee J, Jeong S, et al. Value of imaging study in predicting pelvic lymph node metastases of uterine cervical cancer. Radiat Oncol J 2017; 35(4):340-8. doi: 10.3857/roj.2017.00206.
- Arian A, Easa AM, Arab-Ahmadi M. Diagnostic value of diffusion-weighted magnetic resonance imaging in discriminating between metastatic and non-metastatic pelvic lymph nodes in endometrial cancer. Acta Radiologica 2020; 61(11):1580-6. doi.org/10.1177/0284185120 906660.
- Taylor A, Rockall AG, Reznek RH, Powell MEB. Mapping pelvic lymph nodes: guidelines for delineation in intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2005; 63(5):1604-12. Doi.10.1016/j.ijrobp.2005.05.062.
- Lengelé B, Scalliet P. Anatomical bases for the radiological delineation of lymph node areas. Part III: Pelvis and lower limbs. Radiother Oncol 2009, 92(1):22-33. Doi.10.1016/ j.radonc.2008.11.007.
- Rezaee, A., Jones, J. Endometrioid adenocarcinoma of the endometrium. Reference article, Radiopaedia.org. (accessed on 20 Sep 2022). doi.org/10.53347/rID-35504.
- Takahashi K, Yunokawa M, Sasada S, Takehara Y, Miyasaka N, Kato T, et al. A novel prediction score for predicting the baseline risk of recurrence of stage I–II endometrial carcinoma. J Gynecol Oncol 2019; 30(1):e8. doi:10.3802/ jgo.2019.30.8.