A Rare Case of Post-pubertal Pure Yolk-sac Tumour Incidentally Diagnosed after Surgical Intervention for Fournier’s Gangrene
By Ahmet Asci1, Hakan Bahadir Haberal1, Fariba Amini2, Deniz Ates Ozdemir2, Ahmet Gudeloglu1Affiliations
doi: 10.29271/jcpsp.2022.08.1073ABSTRACT
The testicular tumour is the most common solid malignancy in males between the ages of 15 and 35 years. Testicular tumours most commonly present with a painless testicular mass. Fournier’s gangrene is necrotising fasciitis of the genital, perineal, and perianal region characterized by the microvascular thrombosis and skin necrosis, and is most commonly seen in elderly males with the comorbid conditions. To the best of our knowledge, there is no published case of testicular tumour presenting as Fournier’s gangrene. Herein, we report a case of a young adult male, otherwise healthy, who presented to the emergency room with Fournier’s gangrene and was found to have a metastatic post-pubertal pure yolk-sac tumour of the testis which is extremely rare in the adults.
Key Words: Fournier’s gangrene, Yolk sac tumour, Testicular neoplasm.
INTRODUCTION
Testicular tumours represent the most common solid malignancy in males between the ages of 15 and 35 years and commonly present with a painless testicular mass, dull testicular ache, and rarely with acute testicular pain.1 Fournier’s gangrene (FG) is necrotising fasciitis of the genital, perineal, and perianal region characterised by the microvascular thrombosis and skin necrosis associated with the polymicrobial infection.2 It is more commonly seen in elderly males; diabetes, alcohol abuse, acquired immunodeficiency syndrome (AIDS), or any other disease process compromising host immunity and microcirculation contribute to its development.3 Herein, we present a young male patient, who was admitted to the emergency room (ER) with a massive necrotic scrotal infection/FG. The pathological examination revealed a postpubertal pure yolk-sac tumour (YST) which is extremely rare in adults. To the best of our knowledge, this is the first case of a testicular tumour presenting with FG.
CASE REPORT
A thirty-four years male was admitted to the ER with scrotal pain and discharge. The complaints started approximately 3 months ago as a bullous skin infection of the scrotum and spread from that focus with time. Physical examination revealed a necrotic and ulcerative lesion covering almost the whole scrotum and perineal area, massive enlargement of the scrotum, and widespread purulent discharge from the several sinus tracts. The patient was promptly diagnosed with an advanced case of FG and prepared for the emergency scrotal debridement. Piperacillin-tazobactam 4.5 g IV q6h was commenced.
During exploration, infection was more prominent and spread deeper in the right hemiscrotum (Figure 1). Right testis could not be identified and thus could not be salvaged. The right hemiscrotum and most of the left hemiscrotum were resected in the end leaving only the left testicle and a small amount of healthy skin around it. The wound was irrigated with hydrogen peroxide solution, left to close by secondary intention, and covered with an antibiotic-soaked dressing.
On macroscopic examination, tissue fragments were necrotic and yellowish white (Figure 2). On microscopy, lace-like, tubular, and microcytic appearances were observed at the low power. On high power, tumour cells exhibited eosinophilic to clear cytoplasm with large nuclei and prominent nucleoli. Classical Schiller-Duval bodies were visible (Figure 3). No normal testicular parenchyma could be identified in the specimen, and the spermatic cord showed tumoural infiltration. Widespread lymphovascular invasion and tumour thrombus were seen. In immunohistochemical studies, neoplastic cells were diffusely positive for Alfa-feto protein (AFP) and SALL4. Pure YST diagnosis was made with classical morphologic and immunohistochemical results. Due to the nature of the specimen, surgical margins were difficult to delineate but reported as most probably positive for the disease. The pathology report indicated a minimum pT3 stage based on scrotal invasion status.
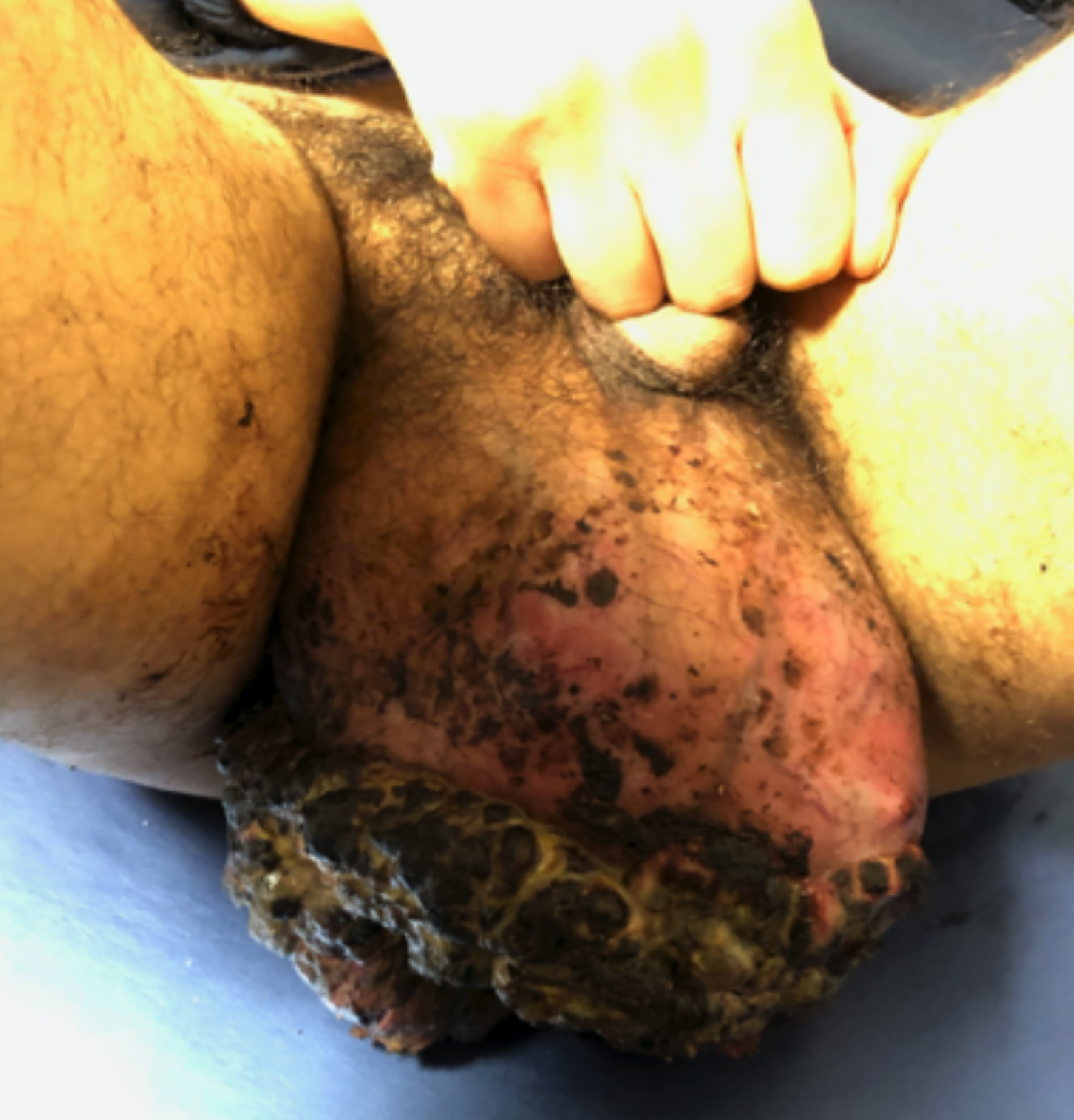 Figure 1: Necrotic infection covering almost the whole scrotum and widespread purulent discharge from the several sinus tracts.
Figure 1: Necrotic infection covering almost the whole scrotum and widespread purulent discharge from the several sinus tracts.
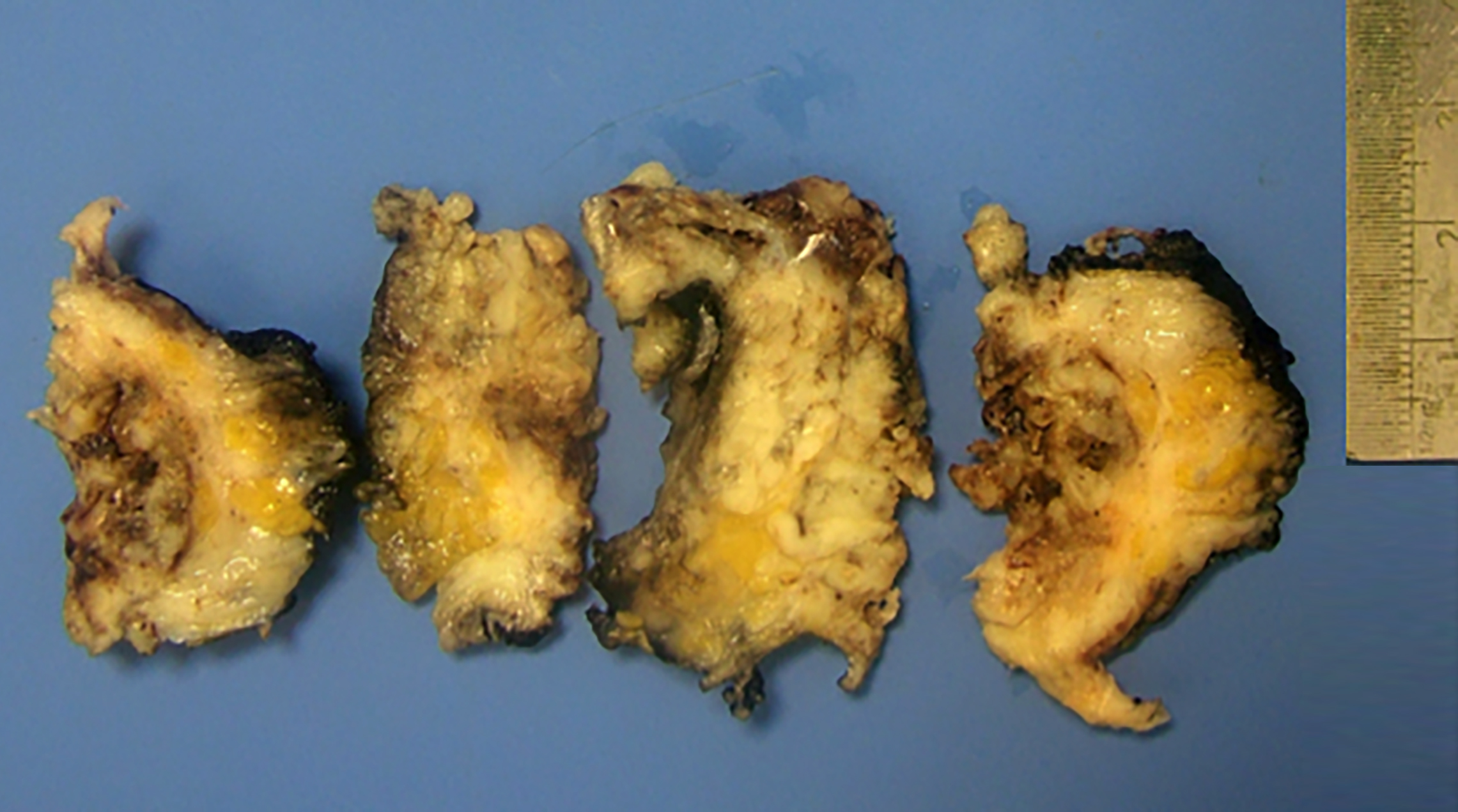 Figure 2: Gross pathological specimen. The cut section is yellow-white and necrotic resembling an abscess wall of the debridement specimen or fat necrosis.
Figure 2: Gross pathological specimen. The cut section is yellow-white and necrotic resembling an abscess wall of the debridement specimen or fat necrosis.
The patient was tested for HIV infection, sexually transmitted diseases (STDs), and syphilis. All were negative. Fasting blood glucose levels ruled out diabetes. Wound culture obtained intraoperatively revealed a polymicrobial infection. Infectious diseases department advised continuation of Piperacillin-Tazobactam regimen.
The blood tests for tumour markers were evaluated on day 5th post-operatively. AFP and B-human chorionic gonadotropin (B-HCG) levels were 102,539 ng/mL and <1,20 mIU/mL, respectively.
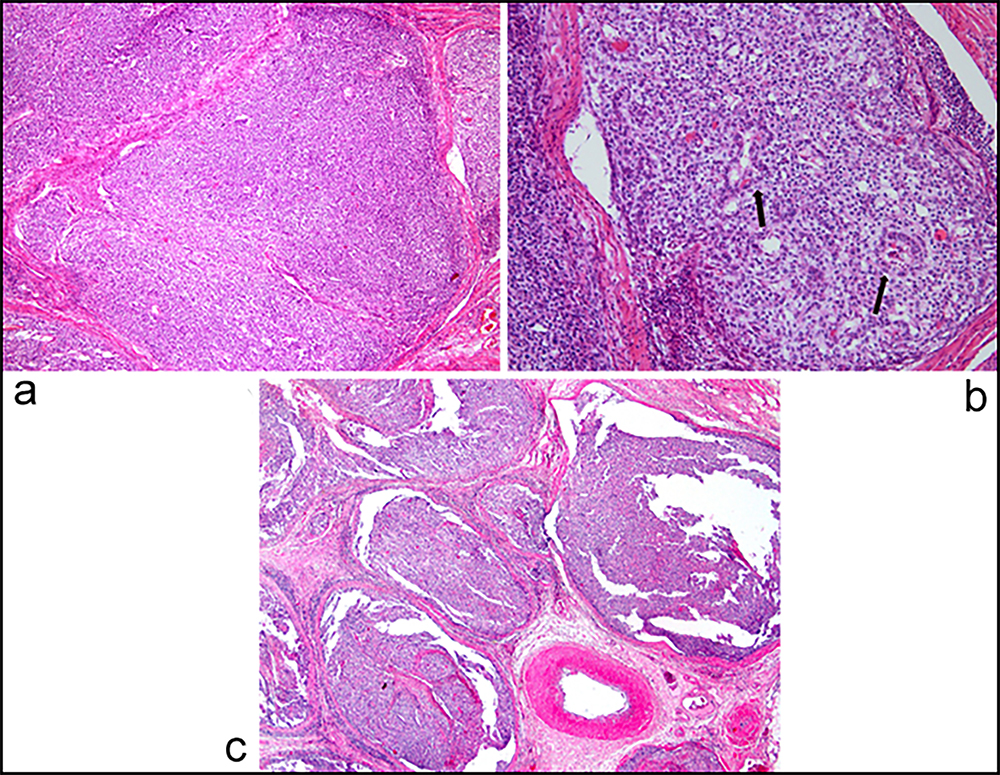 Figure 3: (a) Microcystic, solid, and tubular architecture of the yolk sac tumour (H&E, x 40), (b) Tumour cells with clear and eosinophilic cytoplasm with large nuclei [Schiller-Duval bodies (arrow) can be identified (H&E, 40x)], and (c) vascular spaces are filled with tumour thrombi (H&E, x 100).
Figure 3: (a) Microcystic, solid, and tubular architecture of the yolk sac tumour (H&E, x 40), (b) Tumour cells with clear and eosinophilic cytoplasm with large nuclei [Schiller-Duval bodies (arrow) can be identified (H&E, 40x)], and (c) vascular spaces are filled with tumour thrombi (H&E, x 100).
Computed tomography (CT) of the chest, abdomen, pelvis and head was performed to assess for metastases. CT revealed 17 mm metastatic lesion in the liver. According to the International Germ Cell Cancer Collaborative Group classification, the patient was classified in the poor prognosis group due to the visceral metastasis and AFP levels. Therefore, the patient received a total of 4 cycles of BEP (Bleomycin, Etoposide, Cisplatin) chemotherapy.
The patient was given tumour marker levels and thoraco-abdominal CT in the follow-up period. The patient is now free of disease for 6 months after the chemotherapy with normal levels of tumour markers.
DISCUSSION
FG is commonly seen in elderly males with certain comorbid conditions or risk factors.3,4 Our patient presenting with an advanced stage of FG without any significant medical history which raised questions about compromised host immunity. Rapid testing for HIV and STDs turned out negative. No comorbid conditions or risk factors associated with the development of FG could be identified in this patient. A diagnosis of malignant disease was not suspected during the initial ER evaluation therefore, blood tumour markers were not studied before the surgery. Once pathological examination confirmed the diagnosis of YST, it changed the course of action drastically.
YST is a subtype of non-seminomatous germ cell tumours (NSGCTs).5 YST is mostly seen in the infants and young children, and has a very good prognosis.6 Pure YSTs in adults are extremely rare with <20 published cases.7 YSTs in adults are usually found as a component of mixed NSGCTs. The treatment options after primary orchiectomy for this very rare patient group are not laid out clearly and active surveillance, chemotherapy, and retroperitoneal lymph nodes dissection (RPLND) have all been described in the literature, usually dictated by the surgeon’s preference.8 Foster et al. compared the clinical behavior of stage 1 pure YST to stage 1 NSCGT in adults and found it to be similar.9 However, due to the rare occurrence of these tumours, it may be appropriate to obtain the opinions of experts from urology, oncology, radiology and pathology departments and make a decision about further treatments in multidisciplinary meetings. Our patient having an AFP level >100,000 and non-pulmonary visceral metastasis was placed in the poor prognostic group of NSGCTs. Standard treatment of metastatic NSGCTs in the poor prognostic group is 4 cycles of BEP, and it was commenced in the early postoperative period.
There are a couple of risk factors associated with testis tumour, including cryptorchidism, impaired spermatogenesis, and positive family history.10 The only risk factor in this patient was the history of bilateral orchiopexy at the age of 5 years. When questioned further, he recalled that he felt a round mass in the right testicle about 2 years ago, and this mass grew slowly in time. He never saw a doctor during this time period nor did for 3 months after the infection began. Looking retrospectively, it can be speculated that the testicular mass that had progressed and metastasised within 2 years may have played a role in the patient’s immunocompromised situation that led to FG.
The spontaneous regression of testicular tumours, metastatic or at primary sites, has been observed previously in NSGCTs, and tumour necrosis is thought to be one of the processes involved.11 We speculate that in this case a similar process took place but rather than leading to regression of the mass, extensive tissue necrosis acted as a nidus for infection which in time led to FG.
To conclude, the present case is the first to describe a patient with no predisposing factor for FG, presenting to the emergency room with a massive necrotic scrotal infection. The pathological examination revealed a post-pubertal pure YST. The possibility of testicular tumour should be kept in mind in the presence of any kind of testicular mass including FG in healthy men without comorbid conditions.
PATIENT’S CONSENT:
The patient has given written informed consent to publish his case and images.
COMPETING INTEREST:
The authors declared no competing interest.
AUTHORS’ CONTRIBUTION:
AA, HBH: Made substantial contributions to the conception and design, analysed and interpreted data, performed literature search, and wrote the manuscript.
FA, AG: Made substantial contributions to the analysis, interpretation of data, and revised the draft.
DAO: Made substantial contributions to the conception and design, interpretation of data and revised the draft.
All the authors have approved the final version of the manuscript to be published.
REFERENCES
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 69(1):7-34. doi:10.3322/caac.21551.
- Chennamsetty A, Khourdaji I, Burks F, Killinger KA. Contemporary diagnosis and management of Fournier's gangrene. Ther Adv Urol 2015; 7(4):203-15. doi:10.1177/ 1756287215584740.
- Voelzke BB, Hagedorn JC. Presentation and diagnosis of fournier gangrene. Urol 2018; 114:8-13. doi:10.1016/j. urology.2017.10.031.
- Aridogan IA, Izol V, Abat D, Karsli O, Bayazit Y, Satar N. Epidemiological characteristics of Fournier's gangrene: A report of 71 patients. Urol Int 2012; 89(4):457-61. doi:10.1159/000342407.
- Vasdev N, Moon A, Thorpe AC. Classification, epidemiology and therapies for testicular germ cell tumours. Int J Dev Biol 2013; 57(2-4):133-9. doi:10.1387/ijdb.130031nv.
- Khan S, Jetley S, Pujani M, Neogi S. Pure yolk sac tumour of testis in an adult: A rare occurrence. J Postgrad Med 2014; 60(3):351-3.
- Wang Z, Yan B, Wei YB, Yin Z, Zhou KQ, Yang JR. Adult metastatic yolk sac tumour descending from an intra-abdominal testis: A case report and review of the literature. Oncol Lett 2015; 10(6):3647-50. doi:10.3892/ol.2015.3817.
- Medica M, Germinale F, Giglio M, Stubinski R, Campodonico F, Raggio M, et al. Adult testicular pure yolk sac tumour. Urol Int 2001; 67(1):94-6. doi:10.1159/000050956.
- Foster RS, Hermans B, Bihrle R, Donohue JP. Clinical stage I pure yolk sac tumour of the testis in adults has different clinical behavior than juvenile yolk sac tumour. J Urol 2000; 164(4):1943-4. doi:10.1016/S0022-5347(05)66924-8.
- Rajpert-De Meyts E, McGlynn KA, Okamoto K, Jewett MA, Bokemeyer C. Testicular germ cell tumours. Lancet 2016; 387(10029):1762-74. doi:10.1016/S0140-6736(15)00 991-5.
- Yamamoto S, Hashimoto T, Nojima M, Mori Y, Hirota S, Shima H. Testicular seminoma associated with massive necrosis. Int J Urol 2006; 13(9):1262-3. doi:10.1111/j. 1442-2042.2006.01513.x.