A Histogram Model to Predict the Risk of Bleeding from Oesophageal and Gastric Variceal Rupture in Cirrhosis
By Rubing Liu1, Yurong Sun1, Kewei Xu1, Hongyan Shi1, Shuting Sheng1, Derun Kong2Affiliations
doi: 10.29271/jcpsp.2022.05.586ABSTRACT
Objective: To establish and verify a nomogram for individualized prediction of patients with oesophageal and gastric variceal rupture and haemorrhage in cirrhosis.
Study Design: Descriptive study.
Place and Duration of Study: Department of Digestive Internal Medicine, Funan County People’s Hospital, Anhui, China, from June 2017 to June 2020.
Methodology: Univariate and multivariate logistic regression analyses were used to identify the risk factors for oesophageal and gastric variceal bleeding in cirrhosis. An individualized risk prediction model was established, which was validated by the parallel bootstrap method and an external validation set.
Results: It was found that emotional stimuli (OR=4.591, 95% CI: 1.419-14.852), improper diet (OR=3.702, 95% CI: 1.606-8.526), overwork (OR=3.529, 95% CI: 1.331-9.366), lower temperature (OR=3.013, 95% CI: 1.242-7.308), and increased abdominal pressure (OR=2.416, 95% CI: 0.900-6.487) were independent risk factors for oesophageal and gastric variceal bleeding in cirrhosis. A risk prediction model was established based on the five risk factors, and the R equation test showed that the C-index of the modelling group and the verification group was 0.815 (95% CI: 0.794-0.836) and 0.812 (95% CI: 0.793-0.831), respectively.
Conclusion: The results of the correction curve showed little difference, which indicated that the risk prediction model has good accuracy and differentiation.
Key Words: Cirrhosis, Oesophagus varices and gastric fundus varices, Bleeding, Risk factors, Risk model, Validation.
INTRODUCTION
Acute upper gastrointestinal bleeding is a common complication of the digestive tract system, and the most common cause is oesophageal or gastric fundus varicose vein rupture in decompensated cirrhosis, which can seriously endanger the life of patients.1 Gastric fundus or oesophageal varicose vein rupture is mainly caused by increased portal vein pressure.2 In general, patients with advanced cirrhosis may suffer complications such as gastric fundus or oesophageal varicose veins and increased portal vein pressure.3 Increased portal vein pressure may cause gastric fundus and oesophageal varices, the submucosal small vessel wall will become thinner, and its brittleness will increase, which can lead to bleed. Severe cases may develop massive haematosis, endangering their safety.4
it is difficult to treat oesophageal and gastric variceal bleeding in cirrhosis, which may threaten the life and safety of patients.5,6 Clinical studies have shown that the risk of oesophageal and gastric fundus venous rupture is approximately 30%-50%, and 50% of patients will experience repeated massive bleeding within 1 to 2 years, with a mortality rate of 40%-70%.7 Bleeding does not directly lead to the death of the patient but liver failure and liver coma. In addition, massive bleeding can lead to insufficient blood supply to the brain of the patient, inducing shock, or even threatening the life of the patient.8 Therefore, the analysis of risk factors for bleeding of oesophageal and gastric fundus variceal bleeding in cirrhosis can help medical staffs take targeted treatment and prevention measures to reduce the incidence and mortality of gastrointestinal bleeding and improve the quality of life of patients. The objective of this study was to establish and verify a nomogram for individualized prediction of patients with oesophageal and gastric variceal rupture and haemorrhage in cirrhosis.
METHODOLOGY
A total of 330 patients with oesophageal and gastric fundus varicose veins with cirrhosis admitted to Funan County People’s Hospital, Anhui, China, from June 2017 to June 2020 were selected. The inclusion criteria were patients who met the diagnostic criteria for oesophageal and gastric fundus varicose veins with cirrhosis as specified in “the expert consensus on the diagnosis and treatment of esophageal and gastric fundus varicose vein ravage and bleeding in cirrhosis portal hypertensive patients” (2019 edition); (2) who had not taken octreotide, somatostatin, vasopressin and other portal vein pressure-lowering medicine; who had no other cause of gastrointestinal bleeding. Patients and their family members knew about the study and signed the informed consent form.
The exclusion criteria were severe cardiovascular and cerebrovascular diseases, malignant tumours; nervous system disease, cognitive function and communication barriers; gastrointestinal bleeding caused by other reasons; and patients with incomplete information. This study was approved by the hospital ethics committee.
Data were randomly divided into the training set (n=220) and the validation set (n=110). According to “Guidelines for the diagnosis and treatment of esophageal and gastric variceal bleeding in cirrhotic portal hypertension” (2019 edition), the data were divided into the bleeding group (51 patients with rupture and haemorrhage) with an incidence of 23.18% and the no bleeding group (169 patients without varicose vein rupture and bleeding).
The clinical data of 330 patients with liver cirrhosis oesophageal gastric varices were counted, including gender, age, with or without emotional stimuli evaluated, with self rating anxiety scale, score <53 = normal; 53-62 = mild mood fluctuation; 63-72 = moderate; and >73 = severe, body mass index (BMI), duration, improper diet (raw and cold diet, irritant big and hard food one week before the onset), smoking history, history of alcoholism, with or without overwork (The fatigue scale -14 was used to assess overwork. A score above 10 was defined as overwork, and the higher the score, the more severe the overwork), blood albumin, haemoglobin, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), with or without hypothermia (32~35℃ lasting for at least 24h during treatment), high blood pressure, high cholesterol, and with or without increased abdominal pressure (cough, forcibly defecate, bending the trunk frequently which may increase abdominal pressure. Intra-abdominal pressure whether ≥12 mmHg, was determined by measuring the intra-bladder pressure. The patient was inserted with a three-cavity indwelling catheter. To connect the three-cavity indwelling catheter with a standard pressure sensor, and connect the pressure sensor to a monitoring instrument, a syringe was used to inject 10ml normal saline into the bladder, the sensor was set to zero, flapped on the belly of patient, read the data from the monitoring instrument after 60 seconds).
SPSS 22.0 software was used for analysing the general clinical data of the patients. Baseline characteristics for the randomized population were summarized as mean±SD for quantitative data and as proportions for categorical data. Chi-square test was used for the enumeration data, and an independent-sample t-test was employed for the measurement data. Logistic regression analysis was used to analyse risk factors, and the RMS package and R (R3.5.3) software package were used to construct the histogram model. The caret software package was applied to the bootstrap modelling group for internal validation and the validation group for external validation. The prediction accuracy of the histogram was evaluated by the correction curve and C-index. p<0.05 indicated a significant difference.
RESULTS
Comparative analysis showed that no significant difference between the training and validation sets in terms of gender, age, emotional stimuli, body mass index (BMI), course, diet, smoking, drinking, presence of overwork, blood albumin, haemoglobin, LDL-C, HDL-C, low temperature change condition, high blood pressure, high cholesterol, and the presence of increased abdominal pressure (p >0.05). On univariate analysis, there were no significant differences in terms of gender, age, BMI, disease duration, smoking, alcohol abuse, serum albumin, haemoglobin, LDL-C, HDL-C, and hyperlipidaemia between the two groups (p >0.05), while significant differences were found in emotional stimulation, improper diet, overwork, low temperature and increased abdominal pressure (p <0.05, Table I).
Binary classification logistic regression analysis, taking varicose vein bleeding as the dependent variables and taking the five statistically significant factors obtained from univariate analysis between the haemorrhage group and the no bleeding group (emotional stimuli, improper diet, overwork, lower temperature and increased abdominal pressure) as the independent variables. The results showed that emotional stimuli (OR = 4.591, 95% CI: 1.419 - 14.852), improper diet (OR = 3.702, 95% CI: 1.606 - 8.526), overwork (OR = 3.529, 95% CI:1.331 - 9.366), lower temperature (OR = 3.013, 95% CI: 1.242 - 7.308) and increased abdominal pressure (OR = 2.416, 95% CI: 0.900-6.487) were independent risk factors for oesophageal and gastric variceal bleeding in cirrhosis (p <0.05, Table II).
Based on the five independent risk factors screened out, this study constructed a histographic model to predict bleeding of oesophageal and gastric variceal bleeding in cirrhosis, as shown in Figure 1. Using the bootstrap method, the model was validated internally after repeated sampling of the original data of the training set for 1,000 times, while the validation set was validated externally. The results showed that the C-index of the training set and the validation set were 0.815 (95% CI: 0.794-0.836) and 0.812 (95% CI: 0.793-0.831), respectively. The calibration curve results showed that the standard curve trends of the training set and the validation set were relatively consistent, as shown in Figure 2. The ROC curve results showed that the curve integrals (AUCs) of the training set and the validation set were 0.815 and 0.812, respectively, as shown in Figure 3. The above results indicated that the prediction accuracy of this risk prediction model is high.
Table I: Univariate analysis of oesophageal and gastric variceal bleeding in cirrhosis.
|
Factors |
Haemorrhage group (n=51) |
No bleeding group (n=169) |
p-value |
|
Sex (male, female) |
30(58.8%)/21(41.2%) |
81(47.9%)/88(52.1%) |
0.173 |
|
Age (>60 years / ≤60 years) |
28(54.9%)/23(45.1) |
75(44.4) /94(55.6%) |
0.187 |
|
BMI (kg/m2) |
22.97±2.47 |
23.02±2.89 |
0.782 |
|
Course of disease (years) |
2.19±0.77 |
2.23±1.04 |
0.665 |
|
Smoking (Y/N) |
27(52.9%)/24(47.1%) |
77(45.6%)/92(54.4%) |
0.355 |
|
Alcoholism (Y/N) |
28(54.9%)/23(45.1%) |
72(42.6%)/97(57.4%) |
0.122 |
|
Plasma albumin (g/L) |
39.67±5.60 |
40.14±5.15 |
0.625 |
|
Haemoglobin (g/L) |
107.32±8.38 |
106.93±8.17 |
0.847 |
|
LDL-C (mmol/L) |
2.59±0.42 |
2.63±0.48 |
0.432 |
|
HDL-C (mmol/L) |
2.83±0.43 |
2.79±0.46 |
0.468 |
|
Hyperlipidaemia (Y/N) |
27(52.9%)/24(47.1%) |
78(46.2%)/91(53.8%) |
0.395 |
|
Emotional stimulation (Y/N) |
37(72.5%)/14(27.5%) |
78(46.2%)/91(53.8%) |
0.001 |
|
Improper diet (Y/N) |
29(56.9%)/22(43.1%) |
63(37.3%)/106(62.7%) |
0.013 |
|
Overwork (Y/N) |
30(58.8%)/21(41.2%) |
65(38.5%)/104(61.5%) |
0.010 |
|
Low temperature (Y/N) |
33(64.7%)/18(35.3%) |
66(39.1%)/103(60.9%) |
0.001 |
|
Increased abdominal pressure (Y/N) |
30(58.8%)/21(41.2%) |
72(42.6%)/97(57.4%) |
0.042 |
|
Note: variable assignment, varicose vein rupture and bleeding (Y=1, N=0); emotional stimulation (Y=1, N=0); improper diet (Y=1, N=0); overwork (Y=1, N=0); lower temperature (Y=1, N=0); increased abdominal pressure (Y=1, N=0). |
|||
|
|
|||||
|
|
Regression coefficient |
p-value |
Odds Ratio (OR) |
95% Confidence interval |
|
|
Lower limit |
Upper limit |
||||
|
Emotional stimuli |
1.524 |
0.038 |
4.591 |
1.419 |
14.852 |
|
Improper diet |
1.309 |
0.041 |
3.702 |
1.606 |
8.526 |
|
Overwork |
1.261 |
0.021 |
3.529 |
1.331 |
9.366 |
|
Low temperature |
1.103 |
0.009 |
3.013 |
1.242 |
7.308 |
|
Increased abdominal pressure |
0.882 |
0.013 |
2.416 |
0.900 |
6.487 |
|
Constant |
-7.325 |
<0.001 |
0.001 |
|
|
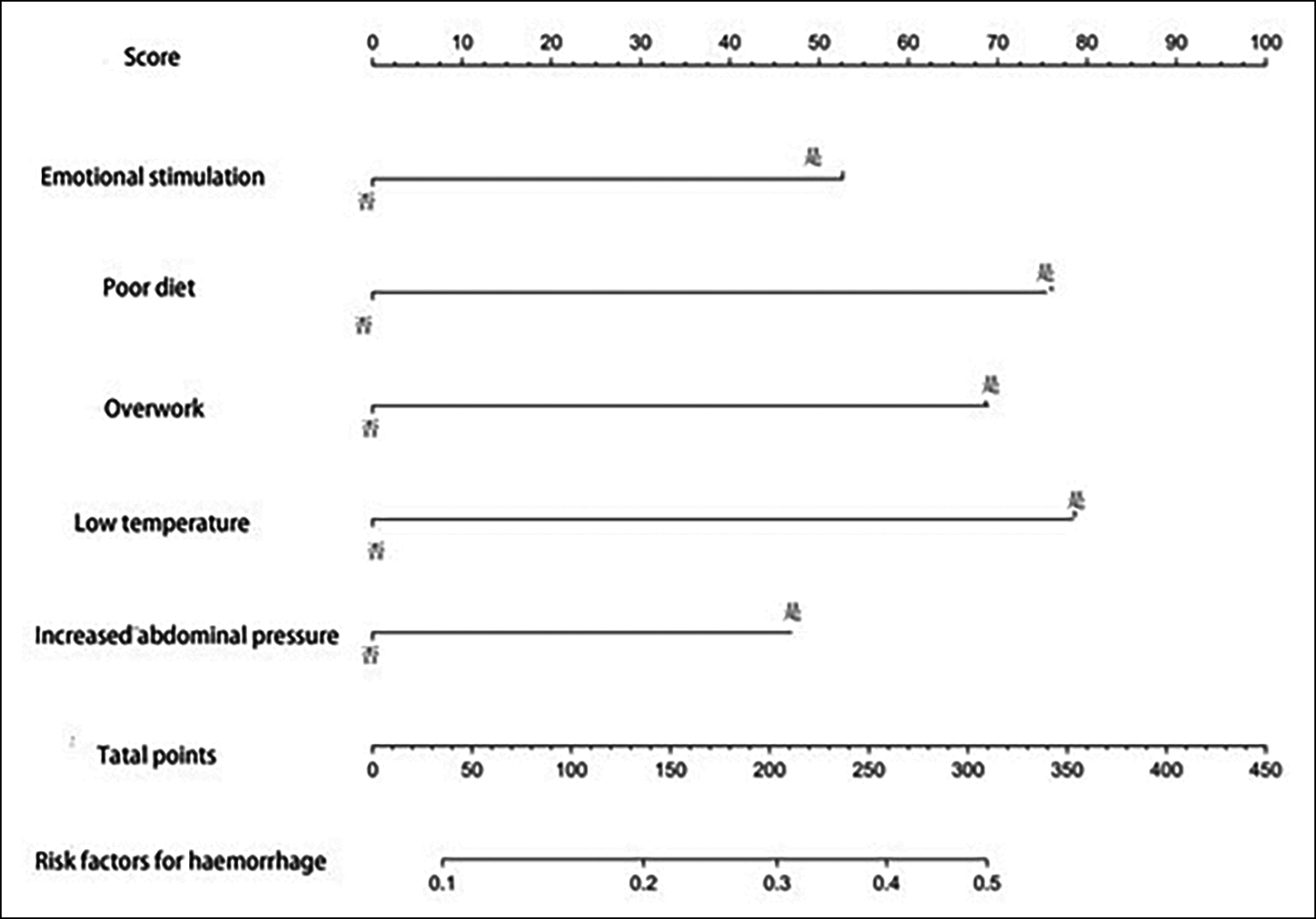 Figure 1: Nomogram for predicting bleeding of oesophageal and gastric fundus varices in cirrhosis.
Figure 1: Nomogram for predicting bleeding of oesophageal and gastric fundus varices in cirrhosis.
DISCUSSION
Cirrhosis is the 8th leading cause of death globally, and liver fibrosis is a dynamic process that is reversible in the early stage.10 Patients with liver cirrhosis usually have clinical manifestations such as portal hypertension and liver function damage in the later stage, which involve multiple organs and systems, and oesophageal and gastric fundus varices are one of the main clinical manifestations of portal hypertension.11 According to clinical studies, bleeding of oesophageal gastric varices can easily occur, bleeding changes haemodynamics, blood volume decreases, the flow changes, cardiac output decreases, blood pressure drops, arterial and venous pressure decreases, and heart rate decreases. Insufficient oxygen leads to insufficient blood perfusion in tissues and hypoxia in related organs and tissues, which leads to functional and morphological impairment and makes the condition more complex.12 In addition, insufficient perfusion may cause metabolic disorders in the body, accumulation of acid metabolites in the body, and difficulty in maintaining high blood vessel tension, increasing capillary permeability and liquid exudation, further changing haemodynamics, aggravating tissue damage, and causing further deterioration of liver function, heart failure and arrhythmia.13 Loss, apathy or restlessness may be caused by reduced ischaemia and hypoxia in cerebral blood supply, and the risk of hepatic encephalopathy will be greatly increased when cerebral blood supply is less than 50%.14 Therefore, the analysis on the influence of ruptured oesophageal gastric variceal bleeding risk will become particularly important, and it has been shown that the associated risk factors mainly include emotional stimuli, improper diet,15 overwork, lower temperature and increased abdominal pressure.15 The authors established an individual risk model based on the risk factors to increase preventive measures for patients with oesophageal gastric vein bleeding.
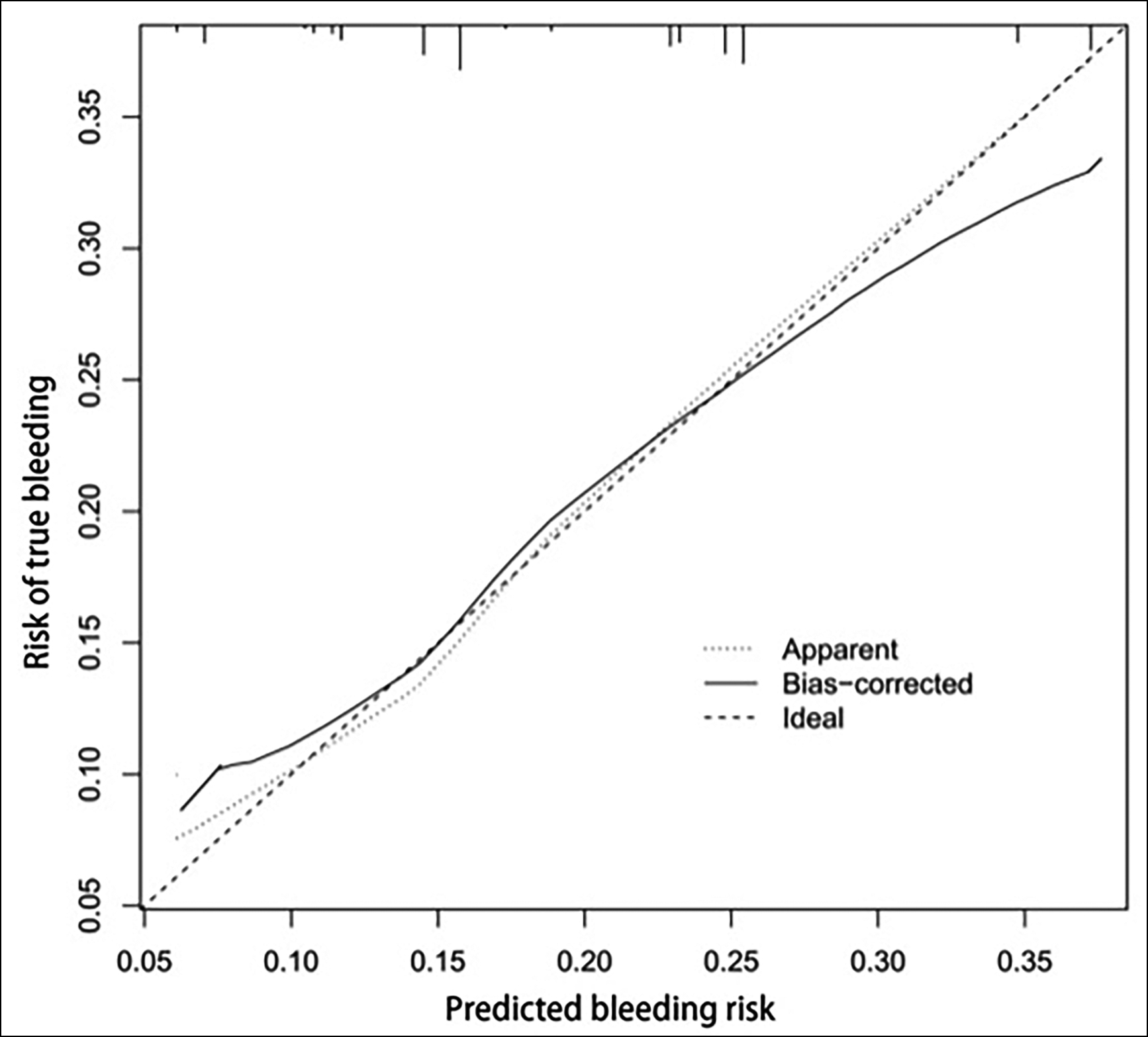 Figure 2: Validation chart.
Figure 2: Validation chart.
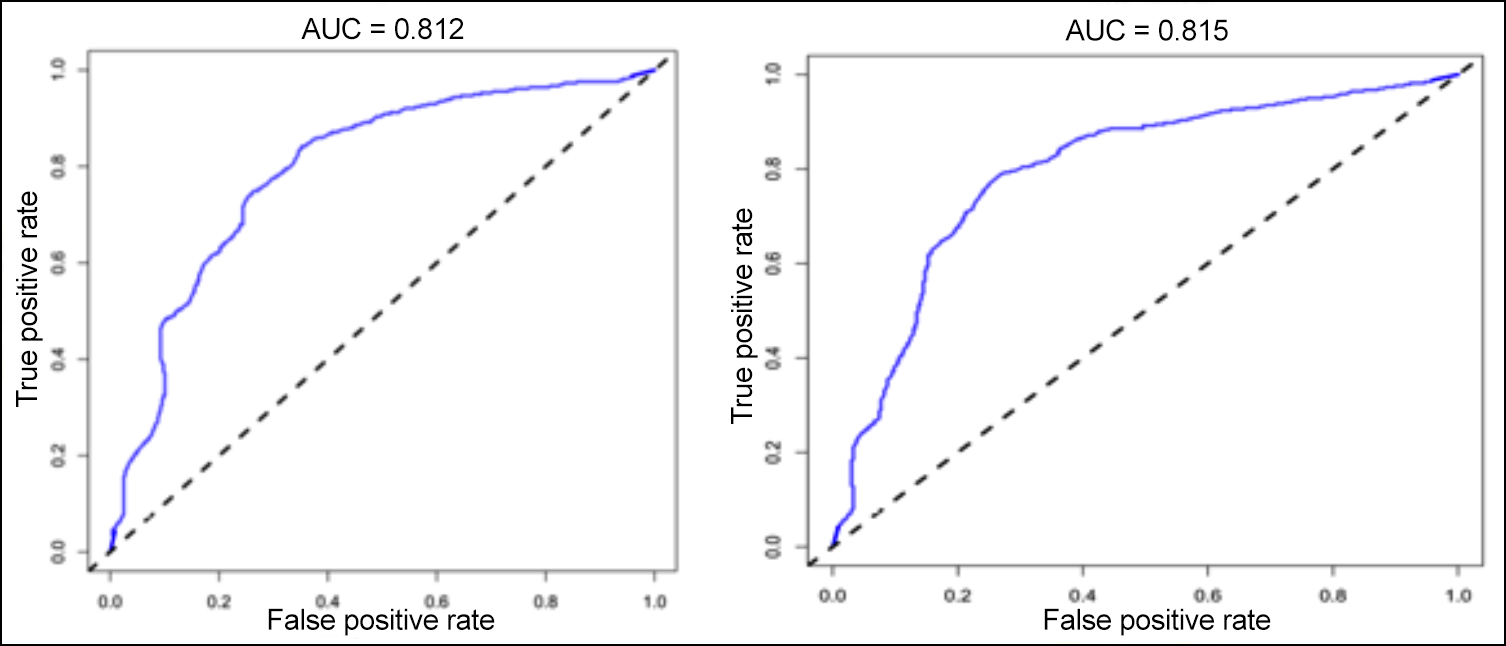 Figure 3: Chart verified by the ROC curve.
Figure 3: Chart verified by the ROC curve.
This study analysed the clinical data of 330 patients with oesophageal and gastric fundus varices with cirrhosis, It showed that emotional stimulation, improper diet, overwork, low temperature and increased abdominal pressure were independent risk factors for oesophageal and gastric fundus variceal bleeding with cirrhosis. In general, with a longer duration of liver disease, the recurrence rate is higher and it is associated with many complications, especially bleeding, which may increase the psychological pressure of patients, leading to more psychological symptoms, such as anxiety, fear, and nervousness. Mental problems, such as these emotional stimuli, can stimulate the sympathetic nervous system to promote excitement, increasing the risk of rupture haemorrhage. In addition, adverse emotional stimulation will aggravate the psychological pressure of patients, and easily induce serious emotions in patients, such as irritability, anger and other strong negative emotions, which may further stimulate the excitability of the sympathetic nervous system and increase the risk of varicose vein bleeding. The results of this study showed that the risk of variceal bleeding induced by emotional stimulation was significantly higher than that of patients with normal emotional stimulation (p <0.05), which was consistent with the results of Subramaniam et al.16
Overeating or eating hard and indigestible food will increase the venous pressure of the patient, in addition to improving the patient's gastrointestinal peristalsis, which may compress the fragile varicose veins, thereby increasing the risk of bleeding. The results of this study showed that the incidence of varicose vein bleeding induced by improper diet was as high as 31.52%, which was an independent risk factor for clinically induced varicose vein bleeding (p <0.05). The research results of Bellis et al. were relatively consistent.17 The function and energy of patients were not sufficient for daily life and work needs, which may aggravate physical fatigue. Excessive fatigue will reduce liver blood flow and reduce the amount of nutrients, such as blood flow and oxygen needed by the body. Liver ischaemia and hypoxia may further reduce liver function, aggravate coagulation dysfunction and increase the risk of varicose vein rupture.18 Environmental temperature also affects variceal rupture haemorrhage, especially early in the morning. At this time, venous blood flow in the heart is at its highest point in patients with a maximum portal venous pressure, thus increasing the risk of rupture haemorrhage. It has been shown that the risk of haemorrhage in patients is higher during the night than during the day, and mortality is higher during the day. Mansour et al. reported that temperature change could also affect portal vein bleeding, which was consistent with the results of this study.19 In addition, it was shown that increased abdominal pressure raised the risk of portal varicose vein bleeding, which may be explained by the fact that increased abdominal pressure will increase portal vein pressure, the liver is located in the abdominal cavity, and hence increased blood pressure will induce the risk of varicose vein bleeding. The sample size of this study was small, and the analysis of influencing factors may be biased to some extent. Retrospective data analysis is another limitation. Therefore, the scope of multicentre studies should be further expanded to comprehensively analyse the risk factors affecting bleeding.
CONCLUSION
Emotional stimulation, improper diet, overwork, low temperature and increased abdominal pressure are independent risk factors for bleeding of oesophageal and gastric fundus varices in cirrhosis. The risk prediction model established in this study can be used to predict the risk of bleeding of ruptured oesophagus and gastric fundus individually and has high clinical value.
ETHICAL APPROVAL:
The study was approved by the Ethics Committee of Funan County People's Hospital (No.20201010, Date: 8/9/2020).
PATIENTS' CONSENT:
Written informed consents were obtained from all patients. The study was conducted in accordance with the principles of the Declaration of Helsinki.
COMPETING INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
RL: The acquisition, analysis, or interpretation of data for the work, drafting the work or revising it critically for important intellectual content.
YS, KX, HS, SS: The acquisition, analysis, or interpretation of data for the work.
DK: Substantial contributions to the conception or design of the work, agreement to be accountable for all aspects of the work in ensuring that question related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
All authors approved the final version of the manuscript to be published.
REFERENCES
- Triantos C, Kalafateli M. Endoscopic treatment of esopha geal varices in patients with liver cirrhosis. World J Gastroenterol 2014; 20(36):13015-26. doi: 10.3748/wjg.v20. i36.13015.
- Dueñas E, Cachero A, Amador A. Ulcer bleeding after band ligation of esophageal varices: Risk factors and prognosis. Dig Liver Dis 2020; 52(1):79-83. doi: 10.1016/j.dld.2019. 06.019.
- Kothari HG, Gupta SJ, Gaikwad NR. Role of non-invasive markers in prediction of esophageal varices and variceal bleeding in patients of alcoholic liver cirrhosis from central India. Turk J Gastroenterol 2019; 30(12):1036-43. doi: 10.5152/tjg.2019.18334.
- Gaecia-Tsao G. Management of varices and cariceal hemorrhage in cirrhosis. New Engl J Med 2010; 362(9):823-32.
- Huber A, Ebner L, Heverhagen JT, Christe A. State-of-the-art imaging of liver fibrosis and cirrhosis: A comprehensive review of current applications and future perspectives. Eur J Radiol Open 2015; 26(2):90-100. doi: 10.1016/j.ejro.2015.05.002.
- Elzeftawy A, Mansour L, Kobtan A. Evaluation of the blood ammonia level as a non-invasive predictor for the presence of esophageal varices and the risk of bleeding. Turk J Gastroenterol 2019; 30(1):59-65. doi: 10.5152/tjg. 2018.17894.
- Binmoeller KF, Endoscopic ultrasound-guided coil and glue injection for gastric variceal bleeding. Endosc Int Open 2019; 7(9):E1061-3. doi: 10.1055/a-0915-9532.
- Stokkeland K, Brandt L, Ekbom A, Hultcrantz R. Improved prognosis for patients hospitalized with esophageal varices in Sweden 1969-2002. Hepatology 2006; 43(3): 500-5. doi: 10.1002/hep.21089.
- Chinese society of hepatology, Chinese medical association, Chinese society of gastroenterology, Chinese medical association, Chinese society of endoscopology, Chinese medical association. Guidelines for the diagnosis and treatment of esophageal and gastric variceal bleeding in cirrhotic portal hypertension. J Clin Hepatol 2016; 32(2): 203-9.
- Flamm SL. Complications of cirrhosis in primary care: Recognition and management of hepatic encephalopathy. Am J Med Sci 2018; 356(3):296-303. doi: 10.1016/j.amjms.2018.06.008.
- Goral V, Yılmaz N. Current approaches to the treatment of gastric varices: Glue, coil application, TIPS, and BRTO. Medicina (Kaunas) 2019; 55(7):335. doi: 10.3390/medicina55070335.
- Sieber C C, Mosca P G, GroszmannR J. Effect of somatostatin on mesenteric vascular resistance in normal and portal hypertensive rats. Am J Physiol 1992; 262 ( 2Pt1): G274-7.
- Xue H, Yuan J, Chao-Li Y, Palikhe M, Wang J, Qiao W, et al. Follow-up study of transjugular intrahepatic portosystemic shunt in the treatment of portal hypertension. Dig Dis Sci 2011; 56:3350-6.
- Paternostro R, Reiberger T, Bucsics T. Elastography-based screening for esophageal varices in patients with advanced chronic liver disease. World J Gastroenterol 2019; 25(3):308-29. doi: 10.3748/wjg.v25.i3.308.
- Mattos ÂZ, Schacher FC, John Neto G. Screening for esophageal varices in cirrhotic patients - Non-invasive methods. Ann Hepatol 2019; 18(5):673-8. doi: 10.1016/j.aohep.2019.06.003.
- Subramaniam R, Niranjan K. Correlation of HVPG level with CTP score, Meld score, ascites, size of varices, and etiology in cirrhotic patients. Saudi J Castroenterol 2016; 22(2): 109-15.
- Bellis L, Nicodemo S, Galossi A, Galossi A, Guarisco R, Spilabotti L, et al. Hepatic venous pressure gradient does not correlate with the presence and the severity of portal hypertensive gastropathy in patients with liver cirrhosis. J Gastrointestin Liver Dis 2007; 16(3):273-7.
- Chen J, Zeng XQ, Mal L, Ma LL, Li B, Tseng YJ, et al. Randomized controlled trial comparing endoscopic ligation with or without sclerotherapy for secondary. Eur J Gastroenterol Hepatol 2016; 28(1):95-100. doi: 10.1097/MEG.000 0000000000499.
- Mansour L, El-Kalla F, El-Bassat H. Randomised controlled trial of scleroligation versus band ligation alone for eradication of gastroesophageal varices. Gastrointest Endosc 2017; 86(2):307-15.