Higher Liver Stiffness Predicting Adverse Outcomes in Cirrhotic Patients with Child-Pugh Grades A and B
By Lin Chunlei1, Ye Qian1, Qu Yundong1, Wang Lei1, Liu Jiaye2, Li Tao1Affiliations
doi: 10.29271/jcpsp.2021.10.1168ABSTRACT
Objective: To describe the baseline characteristics and two-year outcomes of patients with Child-Pugh grades A and B cirrhosis; and evaluate the predictive value of liver stiffness for the development of adverse outcomes (AOs) in this patient population.
Study Design: A prospective cohort study.
Place and Duration of Study: The Second Hospital, Cheeloo College of Medicine, Shandong University, China between September 2018 and March 2021.
Methodology: The present study consecutively included patients with Child-Pugh grades A and B cirrhosis. Patients were followed up every six months until two years. Baseline demographic characteristics and laboratory indexes were collected. Liver stiffness and controlled attenuation parameter were measured at baseline, month 6 and month 12. The observational endpoint was AOs, including liver-related patient death and hepatocellular carcinoma (HCC).
Results: A total of 174 patients were included in the final cohort. Hepatitis B virus (HBV)-induced liver cirrhosis accounted for the vast majority of enrolled cases (82.2%). AOs were observed in six patients. Multivariate logistic regression model was performed and liver stiffness was considered as the only independent predictor for AOs (OR 1.071, p = 0.006). Liver stiffness was also significantly improved at 12 months compared with the baseline data (median 10.6 vs. 13.3 kPa, p <0.001).
Conclusion: Patients with Child-Pugh grades A and B cirrhosis had an acceptable short-term prognosis. Greater liver stiffness predicted two-year AOs in these patients with relatively mild cirrhosis. The prognostic value of changes in liver stiffness warrants further investigation.
Key Words: Liver cirrhosis, Child-Pugh score, Liver stiffness, Asian population, Outcome.
INTRODUCTION
Cirrhosis represents the progression of liver disease and is characterised by portal hypertension, bacterial translocation, and the occurrence of hyperdynamic circulation.1 Cirrhotic patients also experience high mortality rates and negatively affected quality of life, especially in those with decompensated cirrhosis.1,2
The Child-Pugh score has been widely used to distinguish the severity of cirrhosis.3-6 Although several prognostic tools have been applied to identify patients with severe disease, the Child-Pugh score is still considered one of the most important instructive scoring criteria in clinical practice.3-6
Patients with Child-Pugh grade C cirrhosis are characterised by higher mortality rates and worse outcomes. Meanwhile, patients with Child-Pugh score grades A and B are characterised as mild cirrhosis; however, they may also experience adverse events in relatively short periods. The potential risk of disease progression in this patient population is easy to be ignored in clinical practice. Therefore, the baseline characteristics, outcomes, and intuitive predictors of these patients require further clarification. Furthermore, given the differences in various aetiologies of liver cirrhosis between Asian and Western populations7, clinical data from Asia are urgently required.
Liver stiffness assessed, using transient elastography (TE), has been generally acknowledged as an effective non-invasive method to evaluate the degree of liver cirrhosis in clinical practice.8,9 However, the prognostic role of this non-invasive tool in the development of adverse outcomes (AOs) needs further exploration.
The objective of the present study was to describe the baseline characteristics and two-year outcomes of patients with Child-Pugh cirrhosis grades A and B; and to evaluate the predictive value of liver stiffness for AOs.
METHODOLOGY
A prospective cohort study including patients with Child-Pugh grades A and B cirrhosis was performed. Patients were consecutively recruited from the Second Hospital, Cheeloo College of Medicine, Shandong University, China, enrolled between September 2018 and March 2019, and followed-up for at least two years. The protocol for the current study was approved by the Ethics Committee of the Second Hospital, Cheeloo College of Medicine, Shandong University (Approval No. KYLL-2018 (KJ) P-0070), and adhered to the principles of the Declaration of Helsinki. Written informed consents were obtained from all participants. Patients 30 to 75 years of age (including boundary values), diagnosed with cirrhosis in the past six months, were included. Individuals with Child-Pugh grade C cirrhosis, those diagnosed with malignant tumour(s), including hepatocellular carcinoma [HCC] at screening, pregnant women, and those positive for HIV were excluded. The calculation of sample size was based on logistic regression. A sample size of 154 cases was evaluated to be satisfactory with a power of 90% (P0 = 0.10, P1 = 0.21, R2 = 0.00) using software PASS 11.0.7 (NCSS, LLC. Kaysville, Utah, USA).
Cirrhosis was confirmed by liver biopsies, or typical signs of liver cirrhosis, including cirrhosis-related ascites, hepatic encephalopathy, or gastroesophageal variceal bleeding, or typical imaging features of liver cirrhosis and liver stiffness >12 kilo Pascals (kPa), while alanine aminotransferase (ALT) level <200 U/L. Data regarding the following baseline demographic characteristics and laboratory indexes were collected: patient age and gender, aetiology of cirrhosis, ALT, aspartate aminotransferase (AST), total bilirubin (TB) and albumin levels, platelet count (PLT), alpha fetoprotein (AFP), and international normalized ratio (INR). Liver stiffness and controlled attenuation parameter (CAP) were measured using TE (FibroScan, Echosens, Paris, France), according to procedures specified in previous guidelines.8 Liver stiffness was recorded in kPa. A separate case report form (CRF) was generated for each patient, and then the data were collected in an Excel table. The process of data collection were completed by two investigators independently and checked together. Patients were contacted every six months. Follow-up included outpatient consultation and laboratory investigations. TE was performed only at 6 and 12 months.
The Child-Pugh score was calculated, according to a method described in a previous report.10 The observational endpoint of the current study was AOs, including liver-related patient death and HCC. HCC was diagnosed as follows: nodules ≤2.0 cm in diameter and typical imaging features on at ≥2 types of imaging examination (including contrast enhanced ultrasound, contrast enhanced computed tomography, and contrast enhanced magnetic resonance imaging); nodules >2.0 cm in diameter, and typical imaging features in ≥1 one imaging examination; or elevation of AFP level and typical imaging features in ≥1 imaging examination.11
Kolmogorov-Smirnov test was performed to assess the normality of continuous variables. Continuous variables are expressed as mean ± standard deviation (SD) or median (interquartile range [IQR]). The changes of liver stiffness were compared by Wilcoxon signed ranks test. Univariate and multivariate logistic regression models were used to evaluate the predictive value of liver stiffness for AOs in cirrhotic patients. Nonparametric Spearman rank correlation analysis was performed to evaluate the correlation between liver stiffness and other variables. Differences were considered to be statistically significant at p <0.05. Software IBM SPSS version 22.0 (IBM Corp., Armonk, NY, USA) was used for the above analyses.
RESULTS
A total of 179 patients with Child-Pugh grades A and B cirrhosis were screened. Five patients were excluded due to incomplete follow-up; as such, 174 were included in the final cohort study (Figure 1). The mean patient age was 51.9 ± 9.9 years, and most patients (112 cases, 64.4%) were male gender. Hepatitis B virus (HBV)-induced liver cirrhosis accounted for the vast majority of enrolled cases (143 cases, 82.2%). Other main etiologies included alcohol consumption (11 patients, 6.3%), autoimmune (7 patients, 4.0%), and hepatitis C virus (HCV) infection (6 patients, 3.4%).
AOs were observed in six patients, among whom five died due to liver-related events during the two-year follow-up; HCC developed in the other patient. The two-year mortality rate was 2.9% (5/174). Baseline characteristics of the entire patient cohort, AOs, and patients without AOs are summarised in Table I.
Table I: Baseline characteristics of the included patients.
|
|
Total (n = 174) |
Patients with AOs (n = 6) |
Patients (n = 168) |
|
Age (years) |
51.9±9.9 |
53.3±10.7 |
51.9±9.9 |
|
Male (n, %) |
112 (64.4%) |
5(83.3%) |
107(63.7%) |
|
ALT (U/L)* |
24(17-33) |
13(11-27) |
25(17-34) |
|
AST (U/L)* |
27(21-39) |
25(21-45) |
27(21 -39) |
|
TB (umol/L)* |
16.7(12.6-24.7) |
25.8(12.5-92.5) |
16.6(12.5-24.4) |
|
Albumin (g/L)* |
44.3(39.7-47.1) |
41.3(35.9-47.6) |
44.4(39.8-47.2) |
|
Platelet (*109/L)* |
108(70-155) |
110(76-200) |
108(69-154) |
|
AFP (ng/ml)* |
2.6(1.8-4.2) |
1.4(1.1-2.9) |
2.7(1.8-4.3) |
|
INR* |
1.12(1.07-1.22) |
1.20(1.06-1.49) |
1.12(1.06-1.21) |
|
liver stiffness (kPa)*,† |
13.8(9.4-19.9) |
11.8(9.2-64.0) |
13.8(9.4-19.8) |
|
CAP (dB/m)*,† |
225(194-260) |
195(117-244) |
226(197-261) |
|
AOs: Adverse outcomes; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; TB: Total bilirubin; AFP: Alpha fetoprotein; INR: International normalized ratio; CAP: Controlled attenuation parameter. *Mmedian (interquartile range). † Liver stiffness and CAP were performed in 170 and 168 patients, respectively, values of liver stiffness and CAP were available in all patients with AO. |
|||
As data regarding liver stiffness and CAP values were not available for four and six patients, respectively, a total of 168 patients (six with AOs and 162 without AOs) were further analysed using univariate and multivariate logistic regression models (Figure 1). In the univariate model, liver stiffness was a predictor of AOs (odds ratio [OR] 1.044 [95% confidence interval (CI) 1.001–1.089]; p = 0.045). As such, its predictive value was further evaluated. Variables included in the multivariate model included patient age, gender, ALT, TB, AFP, PLT, INR, liver stiffness, and CAP. Liver stiffness was considered to be the only independent predictor of AOs in cirrhotic patients (OR 1.071, 95%CI 1.020–1.125; p = 0.006) (Table II).
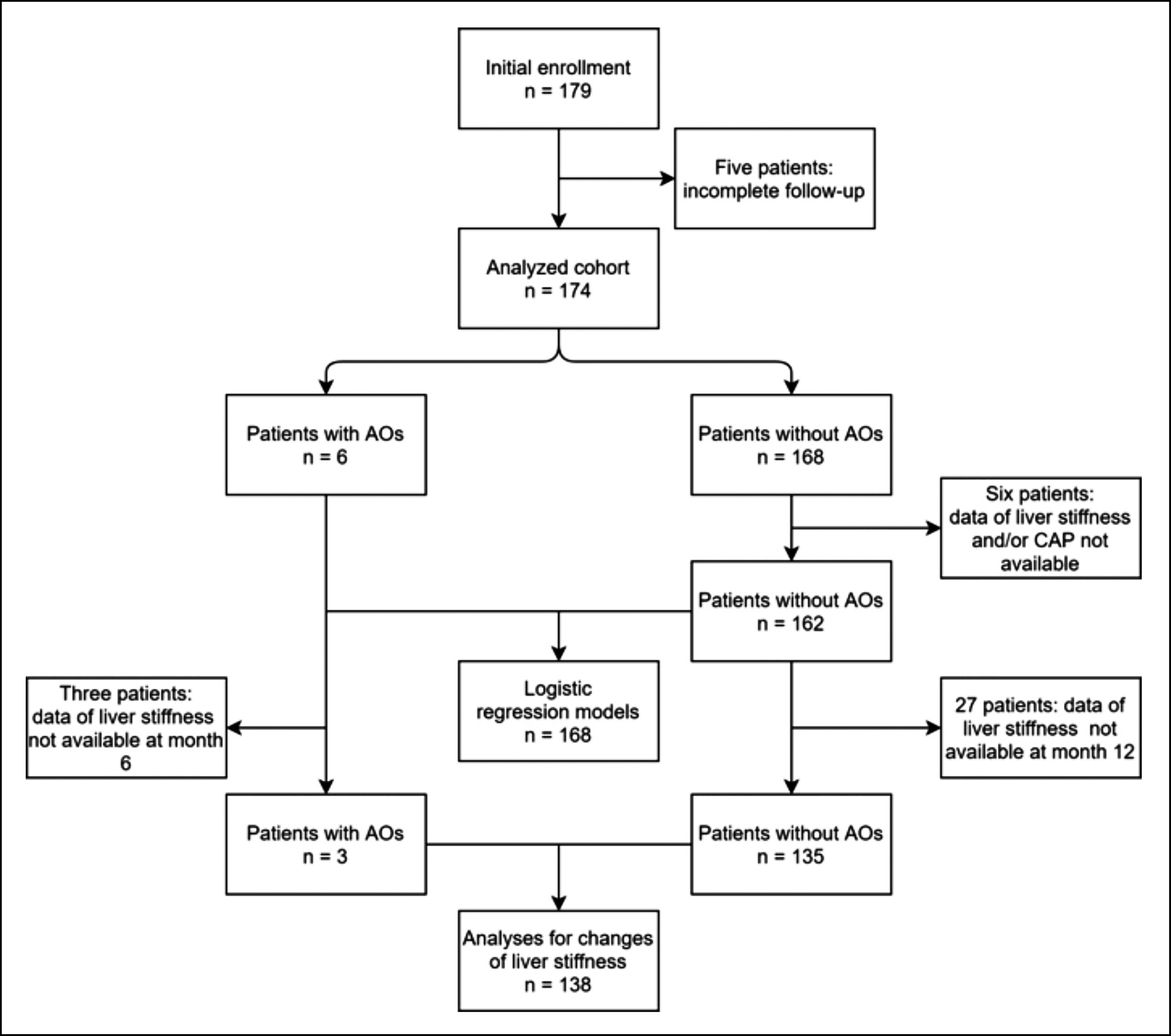 Figure 1: Flow chart of patient inclusion. AOs, adverse outcomes; CAP, controlled attenuation parameter.
Figure 1: Flow chart of patient inclusion. AOs, adverse outcomes; CAP, controlled attenuation parameter.
Bivariate correlation analyses revealed that liver stiffness was correlated with patient age (correlation coefficient [r] = 0.160, p = 0.038), TB (r = 0.201, p = 0.009), PLT (r = -0.238, P = 0.002), and INR (r = 0.229, p = 0.003). No obvious correlation was found between liver stiffness and CAP value or gender (p = 0.233 and 0.190, respectively, Figure 2).
During follow-up, a total of 135 patients without AOs were tested using TE at 6 and 12 months. Liver stiffness data were also available for three patients with AOs (two non-survivors and one HCC patient) at 6 or 12 months (Figure 1). Liver stiffness was significantly improved at 12 months (liver stiffness data in the two non-survivors were obtained at 6 months) compared with baseline data (median 10.6 versus [vs.] 13.3 kPa; p <0.001). Similar results were also obtained for the six patients with alcoholic cirrhosis (median 17.1 vs. 29.5 kPa; p = 0.028), and 117 with HBV-induced cirrhosis (median 9.6 vs. 12.4 kPa; p <0.001). Other aetiologies were not analysed due to the limited sample size.
DISCUSSION
The current study described the baseline characteristics and two-year outcomes of patients with Child-Pugh grades A and B cirrhosis. It was concluded that greater liver stiffness predicted adverse two-year outcomes in these patients with relatively mild cirrhosis. Liver stiffness was also significantly improved after timely treatment.
The outcomes of cirrhotic patients vary depending on disease stage.12 An obvious difference in one-year mortality, from 1% to 57%, was reported by previous literature.12 The aetiology of liver cirrhosis varies greatly across different regions.12
Table II. Exploration of factors related to AOs in patients with Child-Pugh grades A and B cirrhosis.|
|
Univariate |
Multivariate |
||||
|
OR |
95%CI |
p-values |
OR |
95%CI |
p-values |
|
|
Age |
1.015 |
0.934-1.102 |
0.728 |
|
|
|
|
Sex |
0.349 |
0.040-3.060 |
0.342 |
|
|
|
|
ALT |
0.910 |
0.814-1.018 |
0.101 |
|
|
|
|
TB |
1.040 |
0.996-1.087 |
0.076 |
|
|
|
|
AFP |
0.572 |
0.260-1.260 |
0.166 |
|
|
|
|
PLT |
1.003 |
0.991-1.015 |
0.644 |
|
|
|
|
INR |
2.065 |
0.787-5.418 |
0.141 |
|
|
|
|
liver stiffness |
1.044 |
1.001-1.089 |
0.045 |
1.071 |
1.020-1.125 |
0.006 |
|
CAP |
0.987 |
0.974-1.001 |
0.071 |
|
|
|
|
OR: Odds ratio; CI: Confidence interval; AOs: Adverse outcomes; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; TB: Total bilirubin; AFP: Alpha fetoprotein; INR: International normalised ratio; CAP: Controlled attenuation parameter. |
||||||
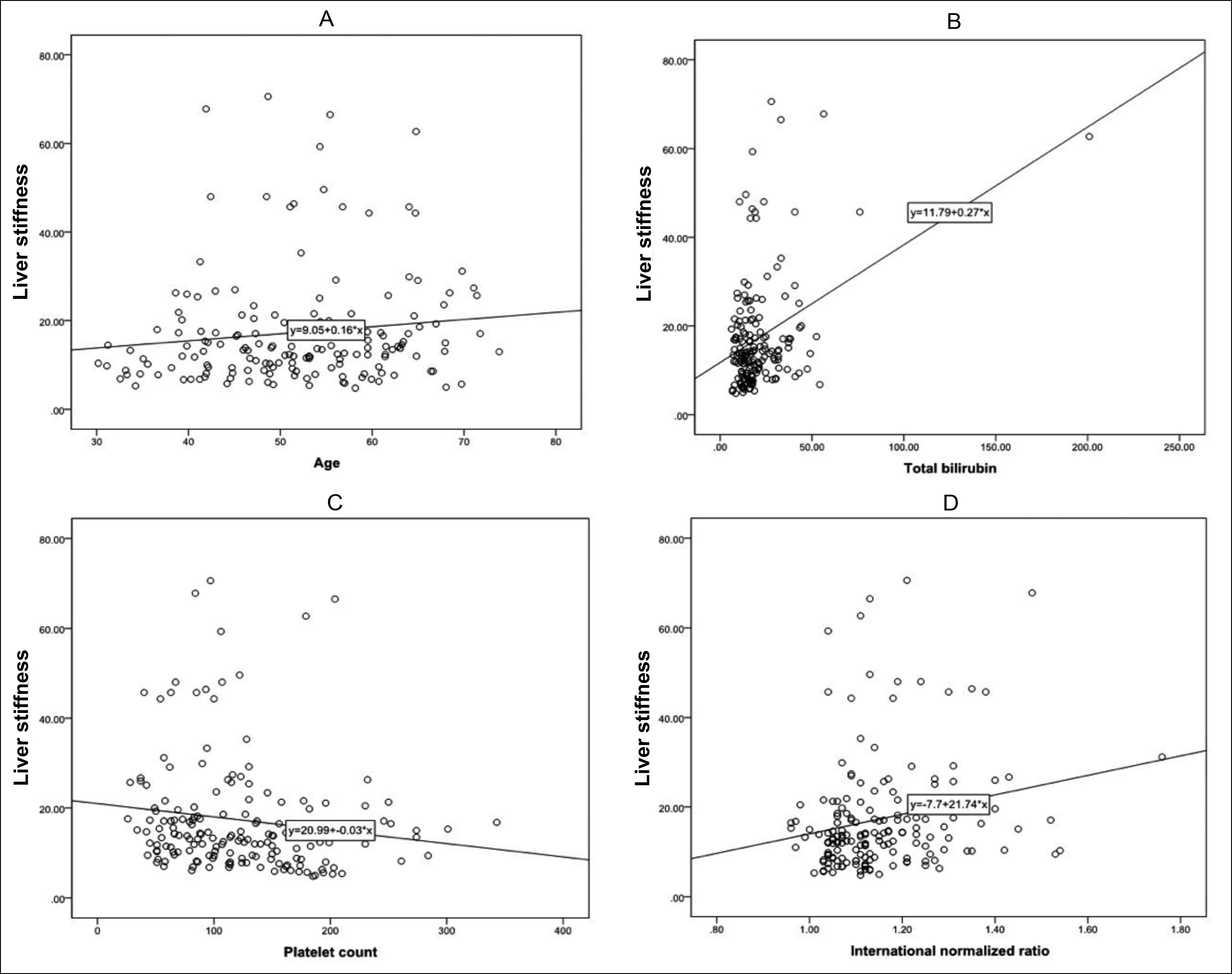 Figure 2: Correlation curves of (A) Liver stiffness and patient age, (B) Liver stiffness and TB, (C) Liver stiffness and PLT, (D) Liver stiffness and INR. TB, total bilirubin; PLT, platelet count; INR, international normalised ratio.
Figure 2: Correlation curves of (A) Liver stiffness and patient age, (B) Liver stiffness and TB, (C) Liver stiffness and PLT, (D) Liver stiffness and INR. TB, total bilirubin; PLT, platelet count; INR, international normalised ratio.
The main causes in Western populations are HCV and alcohol; however, HBV-related cirrhosis accounts for the main cause of cirrhosis in Asian populations.7, 12,13 Unlike previous studies, however, the current cohort focused on Asian patients with Child-Pugh grades A and B cirrhosis. A two-year mortality rate of 2.9% (5/174) was found, and only one case of HCC was screened during the two-year follow-up. The prognosis of patients in the current cohort was obviously better than that of patients in previous studies, especially for those with decompensated cirrhosis,1,7,13-15 illustrating the relatively acceptable outcomes of this subpopulation.
Nevertheless, some patients still progressed to AOs. The acute development of major complications, including ascites, encephalopathy, variceal bleeding, bacterial infection, and kidney dysfunction, are among the main reasons for disease deterioration.1,12,15 Predicting the occurrence of AOs before aggravation of the disease is desirable for this subpopulation. The present study identified liver stiffness as an independent predictor of AOs according to a multivariate logistic regression model. Liver stiffness has been shown to be significantly correlated with liver fibrosis and has been used as an effective noninvasive diagnostic tool for assessing the degree of liver cirrhosis.8,9 Higher liver stiffness values reflect more severe cirrhosis,8 which may be used to provide a more comprehensive judgment of disease. In addition, previous studies performed by our team and other researchers concluded that liver stiffness could also be used to predict esophageal varices and HCC.16,17 Liver stiffness has also been confirmed to be associated with the risk for liver-related events and mortality in patients with nonalcoholic fatty liver disease.18 However, few studies have investigated its value in predicting AOs in patients with compensated cirrhosis of other aetiologies, especially in Asian populations. Therefore, the positive correlation between liver stiffness and progression of cirrhosis explored in the current study is a confirmation of the innovative value of TE and liver stiffness.
Most patients in the present cohort study were also tested for liver stiffness after one year of therapy and demonstrated a significant improvement in liver fibrosis. The above conclusions are consistent with those of previous studies.19,20 A previous study reported a lower risk for HCC in patients with improved liver stiffness.21 Therefore, considering the nature of the cases included in the current cohort, the positive conclusion of the improvement in liver stiffness may provide great inspiration for patients with liver cirrhosis.
The current study also explored changes in liver stiffness in those with cirrhosis of different aetiologies. Patients with HBV-induced cirrhosis, which accounts for the majority of cirrhosis cases in Asian populations, also exhibited findings similar to the total population. However, changes in liver stiffness in patients with cirrhosis of other aetiologies should be further clarified because of the insufficient number of patients in the present cohort.
The present study had some limitations. First, as mentioned above, the sample number of patients with aetiologies other than HBV infection was relatively insufficient. Therefore, the generalisability of these results is limited. Second, the predictive value of changes in liver stiffness for AOs was not explored because of the relatively short follow-up period. Ongoing follow-up of the present cohort study may lead to more valuable conclusions due to its prospective nature and consecutive inclusion of patients with Child-Pugh grades A and B cirrhosis.
CONCLUSION
Patients with Child-Pugh grades A and B cirrhosis had an acceptable short-term prognosis. Greater liver stiffness predicted two-year AOs in these patients with relatively mild cirrhosis. The prognostic value of changes in liver stiffness warrants further investigation.
FUNDING:
This study was funded by Natural Science Foundation of Shandong Province (No. ZR2019PH052), National Natural Science Foundation of China (No. 81803299), Natural Science Foundation of Shandong Province (No.ZR2019PH046), Shandong Medical and Health Science and Technology Development Programs (No. 2015WS0285).
ETHICAL APPROVAL:
The protocol for the current study has been approved by the Ethics Committee of The Second Hospital, Cheeloo College of Medicine, Shandong University (Approval No. KYLL- 2018(KJ) P-0070).
PATIENTS’ CONSENT:
Written informed consents have been obtained from all participants.
CONFLICT OF INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
LT, LJ: Conception and design of the study.
LT, LC, WL, YQ, QY: Patients inclusion and follow-up.
LC, QY: Interpretation of the data.
LT, LJ: Statistical analysis of the data.
LT, LC: Drafted the manuscript.
WL, QY: Revised the manuscript critically.
REFERENCES
- D'Amico G, Morabito A, D'Amico M, Pasta L, Malizia G, Rebora P, et al. Clinical states of cirrhosis and competing risks. J Hepatol 2018; 68(3): 563-76. doi: 10.1016/j.jhep.2017.10.020.
- Acharya C, Bajaj JS. Altered microbiome in patients with cirrhosis and complications. Clin Gastroenterol Hepatol 2019; 17(2):307-21. doi: 10.1016/j.cgh.2018.08.008.
- Peng Y, Qi X, Guo X. Child-pugh versus meld score for the assessment of prognosis in liver cirrhosis: A systematic review and meta-analysis of observational studies. Medicine (Baltimore) 2016; 95(8):e2877. doi: 10.1097/MD.00000000 00002877.
- Xavier SA, Vilas-Boas R, Boal Carvalho P, Magalhães JT, Marinho CM, Cotter JB. Assessment of prognostic performance of albumin-bilirubin, child-pugh, and model for end-stage liver disease scores in patients with liver cirrhosis complicated with acute upper gastrointestinal bleeding. Eur J Gastroenterol Hepatol 2018; 30(6):652-8. doi: 10.1097/ MEG.0000000000001087.
- Salgüero S, Medrano LM, González-García J, Berenguer J, Montes ML, Diéz C, et al. Plasma IP-10 and IL-6 are linked to child-pugh B cirrhosis in patients with advanced HCV-related cirrhosis: A cross-sectional study. Sci Rep 2020; 10(1):10384. doi: 10.1038/s41598-020-67159-3.
- Wan SZ, Nie Y, Zhang Y, Liu C, Zhu X. Assessing the prognostic performance of the child-pugh, model for end-stage liver disease, and albumin-bilirubin scores in patients with decompensated cirrhosis: A large asian cohort from gastroenterology department. Dis Markers 2020; 2020:5193028. doi: 10.1155/2020/5193028.
- Wu T, Li J, Shao L, Xin J, Jiang L, Zhou Q, et al. Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut 2018; 67(12):2181-91. doi: 10.1136/gutjnl-2017-314641.
- European association for study of liver, Asociacion latinoamericana para el estudio del higado. EASL-ALEH clinical practice guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol 2015; 63:237-64. doi: 10.1016/j.jhep.2015.04.006.
- Agbim U, Asrani SK. Non-invasive assessment of liver fibrosis and prognosis: An update on serum and elastography markers. Expert Rev Gastroenterol Hepatol 2019; 13(4):361-74. doi: 10.1080/17474124.2019.1579641.
- Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973; 60(8): 646-9. doi: 10.1002/bjs.1800600817.
- Ayuso C, Rimola J, Vilana R, Burrel M, Darnell A, García-Criado Á, et al. Diagnosis and staging of hepatocellular carcinoma (HCC): Current guidelines. Eur J Radiol 2018; 101: 72-81. doi: 10.1016/j.ejrad.2018.01.025.
- Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet 2014; 383(9930):1749-61. doi: 10.1016/S0140-6736(14) 60121-5.
- Long B, Koyfman A. The emergency medicine evaluation and management of the patient with cirrhosis. Am J Emer Med 2018; 36(4): 689-98. doi: 10.1016/j.ajem.2017.12.047.
- Cao Z, Liu Y, Wang S, Lu X, Yin S, Jiang S, et al. The impact of HBV flare on the outcome of HBV-related decompensated cirrhosis patients with bacterial infection. Liver Int 2019; 39(10):1943-53. doi: 10.1111/liv.14176.
- Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterol 2013; 144(7):1426-37, 37 e1-9. doi: 10.1053/j.gastro.2013.02.042.
- Qu Y, Li T, Ye Q, Zhang L, Wang L. A Beginning or the End? A Meta-analysis to assess the diagnostic accuracy of transient elastography for the prediction of esophageal varices. Saudi J Gastroenterol 2016; 22(5):345-52. doi: 10.4103/ 1319-3767.191138.
- Zhang Y, Wang C, Li H, Ding Y. Decreased liver stiffness by transient elastography indicates lower incidence of hepatocellular carcinoma in patients with chronic hepatitis B. Medicine 2019; 98(3):e13929. doi: 10.1097/MD.0000000 000013929.
- Petta S, Sebastiani G, Viganò M, Ampuero J, Wai-Sun Wong V, Boursier J, et al. Monitoring occurrence of liver-related events and survival by transient elastography in patients with nonalcoholic fatty liver disease and compensated advanced chronic liver disease. Clin Gastroenterol Hepatol 2021; 19(4):806-15.e5. doi: 10.1016/j.cgh.2020.06.045.
- Pons M, Santos B, Simón-Talero M, Ventura-Cots M, Riveiro-Barciela M, Esteban R, et al. Rapid liver and spleen stiffness improvement in compensated advanced chronic liver disease patients treated with oral antivirals. Therap Adv Gastroenterol 2017; 10(8):619-29. doi: 10.1177/1756283 X17715198.
- Elsharkawy A, Alem SA, Fouad R, El Raziky M, El Akel W, Abdo M, et al. Changes in liver stiffness measurements and fibrosis scores following sofosbuvir based treatment regimens without interferon. J Gastroenterol Hepatol 2017; 32(9): 1624-30. doi: 10.1111/jgh.13758.
- Liang LY, Wong VW, Tse YK, Yip TC, Lui GC, Chan HL, et al. Improvement in enhanced liver fibrosis score and liver stiffness measurement reflects lower risk of hepatocellular carcinoma. Aliment Pharmacol Ther 2019; 49(12): 1509-17. doi: 10.1111/apt.15269.