Assessing the Learning Process of Laparoscopic Sleeve Gastrectomy with Different Approaches
By Gokhan Selcuk Ozbalcı, Vahit Mutlu, Ismail Alper TarımAffiliations
doi: 10.29271/jcpsp.2021.11.1331ABSTRACT
Objective: To determine the learning curve (LC) of laparoscopic sleeve gastrectomy (LSG) based on an excess weight loss (EWL).
Study Design: Observational study.
Place and Duration of Study: Ondokuz Mayis University, Faculty of Medicine, Department of General Surgery, from December 2012 to April 2018.
Methodology: Data of patients, who were admitted to the general surgery clinic of a tertiary care hospital and underwent LSG, were retrospectively analysed. Three hundred and twenty-five patients, who had completed at least three months of follow-up after their operations, were included in the study. Patients were divided into three groups according to the number of cases in which the lowest expected EWL values were achieved in the postoperative 3, 6, 12 and 24 months as per literature. Comorbidities, complications, duration of surgery and hospital stay were also evaluated in these groups.
Results: The groups were homogeneous in terms of age and body mass index. Group 3 had a significantly higher median EWL when compared to the other two groups (p <0.001). There was a statistically significant difference between Group 2 and Group 3 in terms of diabetes mellitus and remission of thyroid function tests (p = 0.013 and p=0.017, respectively). There were 40 minutes difference in operating time and two-day difference in hospital stay between the median values of Group 1 and Group 3 (p <0.001).
Conclusion: LSG can be safely performed even in centres that have just started bariatric/metabolic surgical operations. Although proficiency seems to require at least 40 cases, more than 80 operations are needed to complete the LC and achieve ideal results.
Key Words: Bariatric surgery, Learning curve, Metabolic surgery, Laparoscopic sleeve gastrectomy (LSG).
INTRODUCTION
According to the American Society for Metabolic and Bariatric Surgery (ASMBS), 61.4% of operations performed in the United States in 2018 were laparoscopic sleeve gastrectomy operations (LSG). Excluding revision surgeries and procedures such as balloon endoscopy, it was observed that LSG comprised approximately 75% of primary cases.1 LSG is the most preferred method worldwide.2
In addition to the success of LSG in treating obesity and obesity-related comorbidities, the fact that it is technically more straightforward than other procedures and effective, has made it the most preferred type of surgery.3
However, as in all surgeries, the success of LSG directly depends on the care and experience of the surgeon. The assumption that this operation can be performed by every surgeon and lacks standardisation is the main reasons for failure and complications of LSG.4 The importance of the learning curve (LC) concept, defined as the number of times an activity must be repeated before reaching expert status, was first introduced by the German psychologist Hermann Ebbinghaus in 1885.5 The learning curve (LC) of surgical procedures, defined as the number of cases that must be completed to achieve a level of expertise for a particular type of surgery, has been reported as 50–100 cases for LSG per surgeon. Newly established centres consider LC stabilisation after completing 200 cases.3,5,6 However, it should be noted that the learning curve for surgical procedures can be influenced by many factors, such as previous experience and the quality of training received.3
Most previous studies concerning the LC of LSG were not reliable in their significance due to the small number of cases, insufficient follow-up periods, the inclusion of more than one surgeon performing the operations, not being comprehensive enough, or inappropriate statistical analyses.3,6,7
This study aimed to determine the LC of LSG, which is the most preferred bariatric/metabolic surgery (BMS), by evaluating different parameters, especially excess weight loss.
METHODOLOGY
A total of 620 patients, who underwent BMS at the Ondokuz Mayis University, Faculty of Medicine, Department of General Surgery, between December 2012 and April 2018, were retrospectively evaluated. Patients who had BMS surgery other than LSG, and patients who had LSG whose data could not be obtained in the postoperative period were excluded from the study. The first 325 LSG patients who completed two years after the operation and completed at least three months of follow-up, were included in the study. Three hundred and sixteen patients participated in the study with 6-month (m) excess weight loss (EWL), 294 patients with 12-m EWL, and 285 patients with 24-m EWL. Since the two-year postoperative period is considered an early period in BMS, patients who completed at least two years (y) after surgery were included in the study.8 Indications for BMS were decided according to the National Institutes of Health (NIH) 1991 criteria.9
EWL percentage was considered the main parameter of group formation. The lowest expected EWL values needed to be achieved were kept at 31%, 49%, 69%, and 69% at 3, 6, 12, and 24 m (months) postoperatively, respectively.10 The minimum p-value or the so-called maximum statistical approach was used for estimating the cut-off point. Here, for all possible cut points in a given selection range, an appropriate two-sample test is determined with the accompanying test statistic and the p-value (Pc). If any Pc value is less than or equal to the predetermined allowable Type I error level, a cutpoint model may be appropriate. The ideal cutpoint is usually defined as the candidate cutpoint with the smallest Pc.11 The lower limits were determined in the literature for each period by setting the cutoff as the 42nd patient. Accordingly, the first two groups included 42 consecutive cases. All the cases after these two groups were considered the third group. In addition to 3-, 6-, 12-, and 24-m EWL values, the groups were analysed in terms of Diabetes mellitus (DM), hypertension (HT), obstructive sleep apnea syndrome (OSAS), hyperlipidemia (HL), and thyroid function test (TFT) remissions, as well as operating time and length of hospital stay. When calculating operating times, operations that included additional procedures such as cholecystectomy or umbilical hernia repair were excluded, and the remaining 297 patients were included in the analysis. Patients with preoperative comorbidities with available two-year follow-up after surgery (80 DM, 67 HT, 14 OSAS, 54 HL, and 36 patients with abnormal TFT) were included in the evaluation. Remission was classified as complete, partial, or no remission according to the laboratory data and use of drugs (discontinuation or dose reduction). Although major and minor complications were recorded, statistical analysis could not be performed due to the small numbers. Complications requiring reoperation were considered major, while complications that could be followed up were considered minor complications.
The following formula was used to calculate excess weight loss:
Percentage of EWL = (Preoperative weight – Current weight) / (Preoperative weight – Ideal weight) x 100
Miller’s formula was used to calculate ideal body weight.12 Accordingly, fixed body mass index (BMI) was not evaluated, and the ideal weight was calculated separately for each patient.
All surgeries were performed by the same surgeon with advanced laparoscopy experience, but who had recently begun BMS surgeries. The 5-mm trocar technique was used in the reverse Trendelenburg position, and the abdominal cavity was inflated with 13–15 mmHg pressure.
RESULTS
A total of 325 patients (70.8% females and 29.2% males) were included in the study. The mean age was 37 ± 11.25 years, and the mean BMI was 46.5±7.65 Kg/m². One hundred and forty-nine patients had the following comorbidities: DM (24.6%), HT (20.6%), OSAS (4.3%), HL (13.5%) or abnormal TFT (11.1%). In 28 patients, additional surgical procedures were performed. According to the length of hospital stay and availability of the patients, 316 patients participated in the study with 6-m EWL values, 294 patients with 12-m EWL, and 285 patients with 24-m EWL.
Concerning complications, the staple line dehiscence occurred in one patient, and a methylene blue leak was observed in one patient during the operation. Leakage developed in one patient during the postoperative period (Table I). Open surgery was not required in any of the cases.
No significant difference was found between the groups with respect to age and median BMI values (p=0.051 and p=0.082, respectively). There was a significant difference between the median %EWL values of the groups at 3 m, 6 m, 1 y, and 2 y (p <0.001). No significant difference was observed between the median %EWL values of Group 1 and Group 2 at any time. However, median %EWL values were significantly higher in group 3 compared to the other two groups (Figure 1). Median operating time differed among the groups (p <0.001). The median length of hospital stay was six days in Group 1, five days in Group 2, and four days in Group 3. There was no difference in parameters between Groups 1 and 2, whereas Group 3 had significantly lower median values than the other two groups (Table II).
There was a significant difference between the three groups with respect to DM remission (p <0.001) in that 54.5% of the cases in Group 1, 60% of the cases in Group 2, and 88.1% of the cases in Group 3 achieved complete remission. DM remission was significantly higher in Group 3 than in the other two groups (p <0.001). There was no significant difference between the three groups in terms of HT, HL, and TFT remission (p >0.05, Table III).
Table I: Surgical complications.
|
Complications |
Group 1 (n:42) |
Group 2 (n:42) |
Group 3 (n:241) |
Total (n:325) |
|
Major complications |
||||
|
None |
41 (97.6%) |
42 (100%) |
241 (100%) |
324 (99.7%) |
|
Leakage |
1 (2.4%) |
0 (0%) |
0 (0%) |
1 (0.3%) |
|
Minor complications |
||||
|
None |
39 (92.8%) |
40 (95.2%) |
233 (96.7%) |
312 (96%) |
|
Hematoma |
0 (0%) |
0 (0%) |
1 (0.4%) |
1 (0.3%) |
|
Neurologic deficit |
1 (2.4%) |
0 (0%) |
1 (0.4%) |
2 (0.6%) |
|
Portal venous thrombosis |
0 (0%) |
1 (2.4%) |
0 (0%) |
1 (0.3%) |
|
Minor hemorrhage |
1 (2.4%) |
1 (2.4%) |
3 (1.2%) |
5 (1.5%) |
|
Trocar site infection |
0 (0%) |
0 (0%) |
2 (0.8%) |
2 (0.6%) |
|
Intraoperative staple line dehiscence |
1 (2.4%) |
0 (0%) |
0 (0%) |
1 (0.3%) |
|
Intraoperative leak test + |
0 (0%) |
0 (0%) |
1 (0.4%) |
1 (0.3%) |
Table II: Comparison of groups with respect to %EWL.
|
Group 1 (n=42) |
Group 2 (n=42) |
Group 3 (n=241) |
p* |
||||
|
x±σ |
Median (25th percentile-75th percentile) |
x±σ |
Median (25th percentile-75th percentile) |
x±σ |
Median (25th percentile-75th percentile) |
||
|
Age |
40 ± 10.2 |
40 (22 - 63) |
38.7 ± 12 |
39 (18 - 64) |
36.1 ± 11.2 |
35 (17 - 62) |
0.051 |
|
BMI |
49.4 ± 9.2 |
47 (39.4 - 79.6) |
46.4 ± 7 |
44.5 (35.3 - 70.9) |
46.1 ± 7.4 |
44.3 (35 - 73) |
0.082 |
|
3-month %EWL |
35.9 ± 9.5 |
34 (20 - 57.1)a |
40.1 ± 8.4 |
39.9 (25 - 57.1)a |
44.8 ± 10.2 |
43.6 (20.2 - 80.5)b |
<0.001 |
|
6-month %EWL |
50.2 ± 13.8 |
51 (23 - 77.3)a |
56 ± 10.9 |
55.8 (34.2 - 75.3)a |
65.6 ± 14.7 |
63.7 (23.2 - 105.7)b |
<0.001 |
|
1-year %EWL |
61.4 ± 18.2 |
64.1 (20 - 92.4)a |
69.1 ± 13 |
70.5 (43.3 - 90.9)a |
83.3 ± 17.5 |
82.6 (23.2 - 132.5)b |
<0.001 |
|
2-year %EWL |
61.5 ± 24.4 |
62.7 (3.3 - 94.3)a |
69.8 ± 14.7 |
70.2 (46.4 - 95.4)a |
87 ± 18.7 |
86.7 (23.2 - 127.5)b |
<0.001 |
|
Operating time (minutes) |
110.9 ± 16.9 |
110 (75 - 180)a |
102.8 ± 11.2 |
100 (85 - 135)a |
75.9 ± 16.2 |
70 (50 - 180)b |
<0.001 |
|
Hospital stay (days) |
7 ± 4.6 |
6 (4 - 35)a |
5.3 ± 1 |
5 (4 - 8)a |
4 ± 1.1 |
4 (3 - 14)b |
<0.001 |
|
x ̅±σ: mean ± standard deviation, a-b: There was no significant difference between the groups with the same character. *Kruskal Wallis |
|||||||
Table III: Remission of comorbidities after surgery.
|
Group 1 (n=42) |
Group 2 (n=42) |
Group 3 (n=241) |
Total (n=325) |
p* |
|
|
Gender |
|||||
|
Male |
11 (26.2%) |
9 (21.4%) |
75 (31.1%) |
95 (29.2%) |
0.399 |
|
Female |
31 (73.8%) |
33 (78.6%) |
166(68.9%) |
230 (70.8%) |
|
|
DM remission |
|||||
|
Partial |
1 (9.1%) |
3 (30%) |
7 (11.9%) |
11 (13.8%) |
<0.001 |
|
Complete |
6 (54.5%) |
6 (60%) |
52 (88.1%) |
64 (80%) |
|
|
No remission |
4 (36.4%) |
1 (10%) |
0 (0%) |
5 (6.3%) |
|
|
HT remission |
|||||
|
Partial |
5 (29.4%) |
1 (11.1%) |
9 (22%) |
15 (22.4%) |
0.060 |
|
Complete |
7 (41.2%) |
7 (77.8%) |
30 (73.2%) |
44 (65.7%) |
|
|
No remission |
5 (29.4%) |
1 (11.1%) |
2 (4.9%) |
8 (11.9%) |
|
|
OSAS remission |
|||||
|
Complete |
5 (100%) |
8 (88.9%) |
13 (92.9%) |
0 (0%) |
>0.999 |
|
No remission |
0 (0%) |
1 (11.1%) |
1 (7.1%) |
0 (0%) |
|
|
HL remission |
|||||
|
Partial |
1 (14.3%) |
0 (0%) |
7 (18.4%) |
8 (14.8%) |
0.060 |
|
Complete |
2 (28.6%) |
7 (77.8%) |
26 (68.4%) |
35 (64.8%) |
|
|
No remission |
4 (57.1%) |
2 (22.2%) |
5 (13.2%) |
11 (20.4%) |
|
|
TFT remission |
|||||
|
Partial |
4 (66.7%) |
1 (16.7%) |
17 (70.8%) |
22 (61.1%) |
0.056 |
|
Complete |
0 (0%) |
1 (16.7%) |
0 (0%) |
1 (2.8%) |
|
|
No remission |
2 (33.3%) |
4 (66.7%) |
7 (29.2%) |
13 (36.1%) |
|
|
*Chi-square test. |
|||||
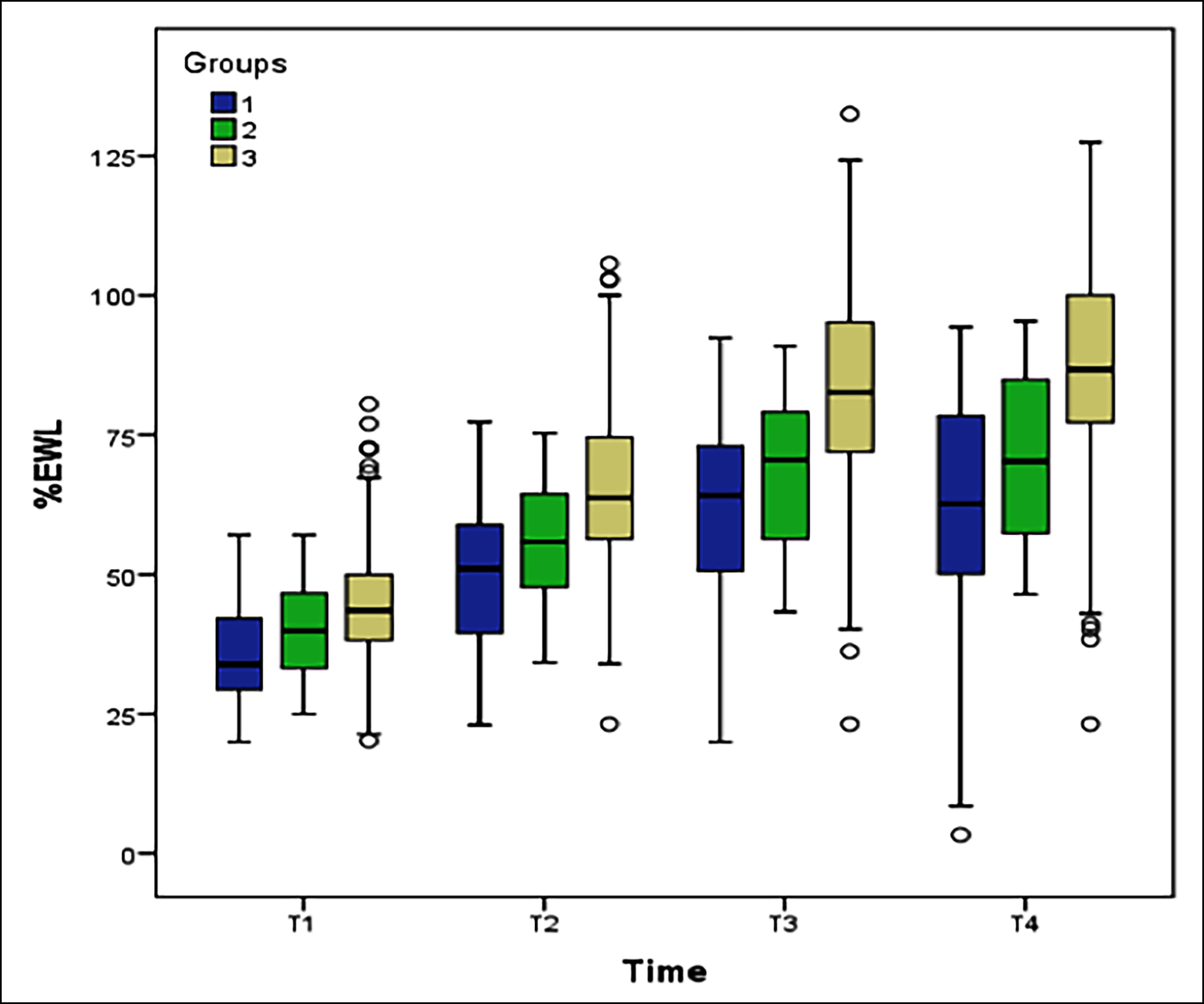
Figure 1: Box plot of % EWL values according to group and time.
DISCUSSION
This study was conducted to detect LC in LSG, the most popular BMS surgery today. The study was carried out on 3 groups formed on the basis of EWL. Common parameters used to evaluate LC in LSG other than weight loss are operation time, conversion to open surgery, discharge time, and complication rates.6,13 In this study, the mean operating time was 84 minutes, and the mean length of hospital stay was 4.5 days, and these results were consistent with the literature.3,5,6 The fact that these durations were different in the third group from the other two groups also supported the approach of the authors. This indicates that a significant decrease in time was achieved in the third group, after about 80 patients.
There was a statistically significant difference between Group 3, considered the period in which LC was completed, and Group 1 in terms of DM, HT, and HL remission, as well as a statistically significant difference between Group 3 and Group 2 in terms of DM and TFT remission. This confirms that assigning groups according to specific criteria (e.g., EWL) is the correct approach, as the surgery is performed correctly. Improvements in comorbidity, such as weight loss rates, will also be positively affected.
There was no significant difference between the groups in terms of minor complications. Bleeding due to hypertension was significantly less in Group 3, as anesthesiologists gradually gain experience in their approach to BMS.
The development of leakage is the most feared complication of LSG and the most significant cause of morbidity and mortality. According to the literature, the incidence of leakage after LSG is 2.4%.14 Studies on LC reported a range between 0–3.9%.3,5,6,15 In the current study, the leak development was observed in only one patient: the 23rd patient.
The number of LSG cases and operating times correlate with postoperative morbidity.16,17 In this LC series, the complication rates were very low, and conversion to open surgery or mortality did not occur. This can be attributed to the operating surgeon’s laparoscopic experience before BMS, performing operations with the same team and selecting appropriate surgical devices.
This study attempted to overcome the shortcomings found in previous studies concerning the rigour in methodology,3,5,6 LC in LSG due to the number of cases,3,6,7,18 follow-up times not being comprehensive enough,3,5,7,18 or inappropriate statistical analysis.3,5,6 Dey, Mittal and Malik published a study evaluating the 6-m follow-up results of an inexperienced team’s initial experience in 50 cases.7 In addition to EWL, comorbidities related to obesity and surgical parameters were also evaluated. However, since this study was an initial-experience study, no LC evaluation was performed as there were no comparative groups.
Moreover, this study had some limitations, such as a small number of cases and a short follow-up period. The study by Zacharoulis et al. is one of the first studies in this area on LC in the first 102 LSG cases.6 However, they divided 102 patients into 3 consecutive groups with an equal number of patients and assigned the groups. As a result, they determined LC as the 68th case. The operation times and hospital stay were similar to this study. However, the difference in follow-up times between the groups [mean = 19 months (1-41)] was a handicap in this study and may limit the results. In addition, since the cases of two surgeons were evaluated, the results were likely to be affected by the skill and experience differences of the surgeons. The study by Carandina et al. evaluated parameters such as weight loss, comorbidities, operating time, intraoperative complications, discharge time, and the number of staples used in operations, which reflected the economic effects of LC in LSG.3 The study consisted of 99 patients followed up for one year after surgery, and the patients were divided into 3 consecutive groups. As a result, LC was reported as near 60 cases. The study was similar to our study in that it included cases belonging to the same surgeon. In addition, the duration of operation and hospital stay were similar to this study. However, the assignment of groups, the limited number of cases, and the short follow-up period were the study limitations. Especially, the lack of difference between the groups in comorbidities, may be due to the small number of cases and the short follow-up periods. The studies by Fantola et al., which followed 110 patients for one year and shared the experiences of a single surgeon who had laparoscopy experience but who just started bariatric surgery, were similar to ours in this respect. Operation times and hospital stays were also similar to this study. However, it was a handicap that the evaluation parameters were low and especially comorbidities were not evaluated. Moreover, the number of patients and short follow-up periods appeared as handicaps, too.18 Major .divided their series of 500 cases into 5 groups and stated that LC stabilisation could extend to the 200th case.5 The fact that the follow-up periods were not specified, the method by which the analysed groups were formed, and the fact that obesity-related comorbidities were not evaluated among the groups were the handicaps of this study. However, the high number of patients, the detailed examination of post-surgical morbidities, and the fact that LC was not defined as a single breakpoint but as a range were similar aspects to this study.
This study aimed to fill the gap in the literature by evaluating the learning curve in LSG with an objective statistical approach. When evaluating LC, it is crucial to ensure a sufficient number of patients who have completed a standard follow-up period and then analyse the results using an appropriate statistical method. In BMS, the first two years after surgery is defined as an early-term, 2–5 years as mid-term, and ≥ 5 years as long-term.8 Even in the best practices in the United States, only 90% of the patients can be followed up for one years.8 Keeping this in view, the strength of our study was that 88% of patients completed the two-year follow-up.
In this study, when determining patient groups, improvement in EWL was considered the primary determinant of BMS success. The authors did not randomly divide the cases into consecutive groups. The cutoff point was determined according to the lowest EWL values, which should have been achieved according to the literature. It is statistically incorrect to create groups by dividing the total number of cases by random numbers without adhering to any criteria. Statistical decision-making processes can be grouped under two basic frameworks, objectively and subjectively. While subjective decision-making processes are based on assumptions or personal opinions, objective decision-making processes are based on statistical observations. When evaluated in this context, the decision-making process based on statistical observation in determining the best cut-off points reveals the strength of this study.
In the present study, based on the definition of LC for a single surgeon, the authors preferred to evaluate the learning curve through a single surgeon who had experience in laparoscopy but has just started bariatric metabolic surgery. The experience and skill of a single surgeon are likely to affect the results; this could be a weak point of the study. However, in studies involving multiple surgeons, the differences in experience and skills of surgeons may be a factor affecting the results.
Although the minimum expected EWL values defined in the literature were found in the second group, the most statistical difference was observed in the third group. When obesity-related comorbidities, operation time and hospital stay were evaluated together, it can be seen that the main difference was in the 3rd group. Instead of determining a single breaking point with the correct statistical approach, it is possible to classify the learning curve as inexperienced, moderately experienced, and experienced periods. Therefore, the first 40 cases were considered as the inexperienced period, the subsequent 40 cases as moderate experience, and ≥80 cases as the experienced period.
CONCLUSION
Laparoscopic sleeve gastrectomy is a procedure that can be safely applied by surgeons with laparoscopic. However, a certain level of experience is needed. Although proficiency seems to require, 40 cases, ensuring standardisation and achieving ideal results, take place after approximately 80 cases. Afterwards, there is a significant decrease in the operating time and hospitalisation time as well as significant improvement in weight loss and comorbidities.
ETHICAL APPROVAL:
Since it was designed as a retrospective study, the data were collected from the hospital archive after approval of the Ethics Committee.
PATIENTS’ CONSENT:
Not applicable.
CONFLICT OF INTEREST:
The authors declared no conflict of interest.
AUTHORS’ CONTRIBUTION:
GSO, VM, IAT: Conceived the study design, involved in data collection, performed the statistical analysis, interpreted data, and prepared the manuscript draft.
All the authors critically reviewed the final version of the manuscript and approved it for publication.
REFERENCES
- American society for metabolic and bariatric surgery. Estimate of Bariatric Surgery Numbers, 2011-2019. Available from: https://asmbs.org/resources/estimate-of- bariatric-surgery-numbers.
- Kehagias I, Zygomalas A, Karavias D, Karamanakos S. Sleeve gastrectomy: Have we finally found the holy grail of bariatric surgery? A review of the literature. Eur Rev Med Pharmacol Sci 2016; 20(23):4930-42.
- Carandina S, Montana L, Danan M, Zulian V, Nedelcu M, Barrat C. Laparoscopic sleeve gastrectomy learning curve: Clinical and economical ımpact. Obes Surg 2019; 29(1):143-8. doi: 10.1007/s11695-018-3486-3.
- Peterli R, Borbély Y, Kern B, Gass M, Peters T, Thurnheer M, et al. Early results of the Swiss Multicentre Bypass or Sleeve Study (SM-BOSS): A prospective randomised trial comparing laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass. Ann Surg 2013; 258:690–4. doi: 10.1097/SLA. 0b013e3182a67426.
- Major P, Wysocki M, Dworak J, Pędziwiatr M, Pisarska M, Wierdak M, et al. Analysis of laparoscopic sleeve gastrectomy learning curve and ıts ınfluence on procedure safety and perioperative complications. Obes Surg 2018; 28(6):1672-80. doi: 10.1007/s11695-017-3075-x.
- Zacharoulis D, Sioka E, Papamargaritis D, Lazoura O, Rountas C, Zachari E, et al. Influence of the learning curve on safety and efficiency of laparoscopic sleeve gastrectomy. Obes Surg 2012; 22(3):411-5. doi: 10.1007/s11695-011- 0436-8.
- Dey A, Mittal T, Malik VK. Initial experience with laparoscopic sleeve gastrectomy by a novice bariatric team in an established bariatric center--a review of literature and initial results. Obes Surg 2013; 23(4):541-7. doi: 10.1007/s11695- 012-0797-7.
- Courcoulas AP, Schauer PR. The Surgical Management of Obesity. Brunicardi FC. Schwartz Principles of Surgery. 11th edition. McGraw-Hill Education. 2019; p. 1167-1218.
- NIH conference. Gastrointestinal surgery for severe obesity. Consensus development conference panel. Ann Intern Med 1991; 115(12):956-61.
- Osland E, Yunus RM, Khan S, Memon B, Memon MA. Weight loss outcomes in laparoscopic vertical sleeve gastrectomy (LVSG) versus laparoscopic roux-en-y gastric bypass (LRYGB) procedures: A meta-analysis and systematic review of randomised controlled trials. Surg Laparosc Endosc Percutan Tech 2017; 27(1):8-18. doi: 10.1097/SLE.00000 00000000374.
- Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using “optimal” cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst 1994; 86(11): 829-35. doi: 10.1093/ jnci/86.11.829.
- Miller MA. A calculated method for determination of ideal body weight. Nutr Support Serv 1985; 5(3):31-3.
- Casella G, Soricelli E, Giannotti D, Bernieri MG, Genco A, Basso N, et al. Learning curve for laparoscopic sleeve gastrectomy: Role of training in a high-volume bariatric center. Surg Endosc 2016; 30(9):3741-8. doi: 10.1007/ s00464-015-4670-3.
- Aurora AR, Khaitan L, Saber AA. Sleeve gastrectomy and the risk of leak: A systematic analysis of 4,888 patients. Surg Endosc 2012; 26(6):1509-15. doi: 10.1007/s00464-011- 2085-3.
- Benaiges D, Más-Lorenzo A, Goday A, Ramon JM, Chillarón JJ, Pedro-Botet J, et al. Laparoscopic sleeve gastrectomy: More than a restrictive bariatric surgery procedure? World J Gastroenterol 2015; 21(41):11804-14. doi: 10.3748/wjg. v21.i41.11804.
- Reames BN, Bacal D, Krell RW, Birkmeyer JD, Birkmeyer NJ, Finks JF. Influence of median surgeon operative duration on adverse outcomes in bariatric surgery. Surg Obes Relat Dis 2015; 11(1):207-13. doi: 10.1016/j.soard.2014.03.018.
- Birkmeyer JD, Finks JF, O'Reilly A, Oerline M, Carlin AM, Nunn AR, et al. Michigan bariatric surgery collaborative. Surgical skill and complication rates after bariatric surgery. N Engl J Med 2013; 369(15):1434-42. doi: 10.1056/NEJMsa1300625.
- Fantola G, Agus M, Runfola M, Rebecchi F, Podda C, Moroni R. Analysis of the learning process for laparoscopic sleeve gastrectomy: CUSUM-curve of 110 consecutive patients with 1-year follow-up. J Visc Surg 2020; 20:1878-7886. doi: 10.1016/j.jviscsurg.2020.05.001.